CUHK
News Centre
Breakthrough by CUHK Engineering in Battery ResearchElectrolyte Made with Skin Cream Ingredients Enables Stable and Non-flammable Aqueous Li-ion Batteries
A research team led by Prof. Yi-Chun LU from the Faculty of Engineering at The Chinese University of Hong Kong (CUHK) has taken a critical step forward in improving high-energy batteries by introducing a novel electrolyte to the aqueous lithium-ion (Li-ion) battery. This electrolyte is commonly used in skin cream. It is inexpensive, non-flammable, less toxic and is eco-friendly, yet can create stable voltage for common usage. The breakthrough was recently published in the world-leading scientific journal, Nature Materials, a sister journal of Nature.
The transformation of the Li-ion battery: from flammable organic to aqueous
In electronic devices and gadgets such as cell phones and laptops which have improved our daily lives on many levels, you can always find a Li-ion battery. As a result of the rechargeable characteristics and stable energy output, they have become the heart of these electronics. Despite years of research, Li-ion batteries still heavily rely on toxic and flammable organic electrolytes to produce power, and the serious safety hazards remain unsolved. The Samsung smartphone explosions and batteries on fire in a novel Boeing plane are just a few examples. These incidents have indicated that the safety of Li-ion batteries is not yet assured.
In contrast, aqueous Li-ion batteries are non-flammable and because of their water-based electrolyte, they do not pose any significant risks of explosion. However, aqueous Li-ion batteries have been suffering from low energy-density issues due to the low battery cell voltage limited by water stability. Electrolysis will occur and break down water into hydrogen and oxygen when the voltage is over 1.23 Volts, destabilising the battery operation and voltage output. Existing approaches to increase the cell voltage of aqueous batteries often involve the use of a large amount of expensive and toxic Li-ion salts to stabilise water molecules, which raises the issues of cost, toxicity and environmental sustainability.
Skin cream ingredient helps stabilise aqueous electrolyte
Building on their previous research on aqueous Li-ion batteries, Prof. LU’s team introduced “molecular crowding” into a novel aqueous electrolyte to replace Li-ion salts as the main stabilisation agent.
“Molecular crowding” is a common phenomenon in living cells, describing the fact that the properties of solution molecules can be substantially modified when macromolecules (proteins, complex sugars, polysaccharides and so on) or small hydrophilic molecules (metabolites, osmolytes) reach a certain concentration level. As a consequence of crowded environments, the activity of the water solvent is reduced owing to the changes in the water’s hydrogen-bonding structuring.
Prof. LU’s team chose to use poly(ethylene glycol) or PEG to replicate “molecular crowding” in the electrolyte. PEG is a water soluble polymer that can be easily incorporated into aqueous batteries. It is also the basis of many skin creams and personal lubricants and is even used in toothpastes and as an anti-foaming agent in food and drinks.
Using this novel stabilisation agent, a LiMn2O4 cathode and a Li4Ti5O12 anode, the team successfully expanded the aqueous electrolyte stability window to 3.2 Volts and demonstrated stable battery operation for delivering a high energy density of 75-100Wh per kg over 300 cycles using this new electrolyte; the operational voltage can be further improved to over 4.0 Volts with gel coating. The common side reactions in aqueous Li-on batteries (hydrogen / oxygen evolution reactions) are virtually eliminated. A flammability test was conducted to prove the fireproof characteristics of the novel electrolyte, significantly improving the safety of Li-ion batteries.
Prof. LU said, “This electrolyte enables the use of many electrode materials that cannot be used in the conventional aqueous electrolytes. More importantly, this research finding provides a new platform for designing an aqueous electrolyte with large-voltage window and high stability for safe, low-cost and eco-friendly energy storage.”
About Prof. Yi-Chun LU
Prof. Yi-Chun LU received her B.S. degree in Materials Science & Engineering from the National Tsing Hua University, Taiwan, in 2007. She received her Ph.D. degree in Materials Science & Engineering from the Massachusetts Institute of Technology (MIT), Cambridge, USA in 2012. Prof. LU has been a research affiliate of MIT since 2013. She is currently an Associate Professor in the Department of Mechanical and Automation Engineering at CUHK. She was awarded China’s Excellent Young Scientists Fund 2019 for her research work on electrochemical energy storage and material interface science. It is her goal to develop a stable, low-cost, scalable and rechargeable energy storage system.
Prof. LU is a Founding Member of the Young Academy of Science of Hong Kong and Associate editor of Journal of Materials Chemistry A published by the Royal Society of Chemistry. She has had conferred on her various CUHK and international research and teaching awards, including Young Researcher Award (2016), the University Education Award, CUHK (2016), the Vice-Chancellor’s Exemplary Teaching Award, CUHK (2014), the Early Career Award, Research Grant Council, Hong Kong SAR (2014), Massachusetts Institute of Technology Martin Family Society of Fellows for Sustainability (2009) and the Taiwan National Science Council Outstanding Research Innovation Award (2007).
CUHK Faculty of Engineering has produced a video to demonstrate the production of the electrolyte, fireproof and energy test of the novel aqueous Li-ion Battery. You may view it here.
Reference:
Xie, J., Liang, Z. & Lu, Y. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. (2020).
https://doi.org/10.1038/s41563-020-0667-y
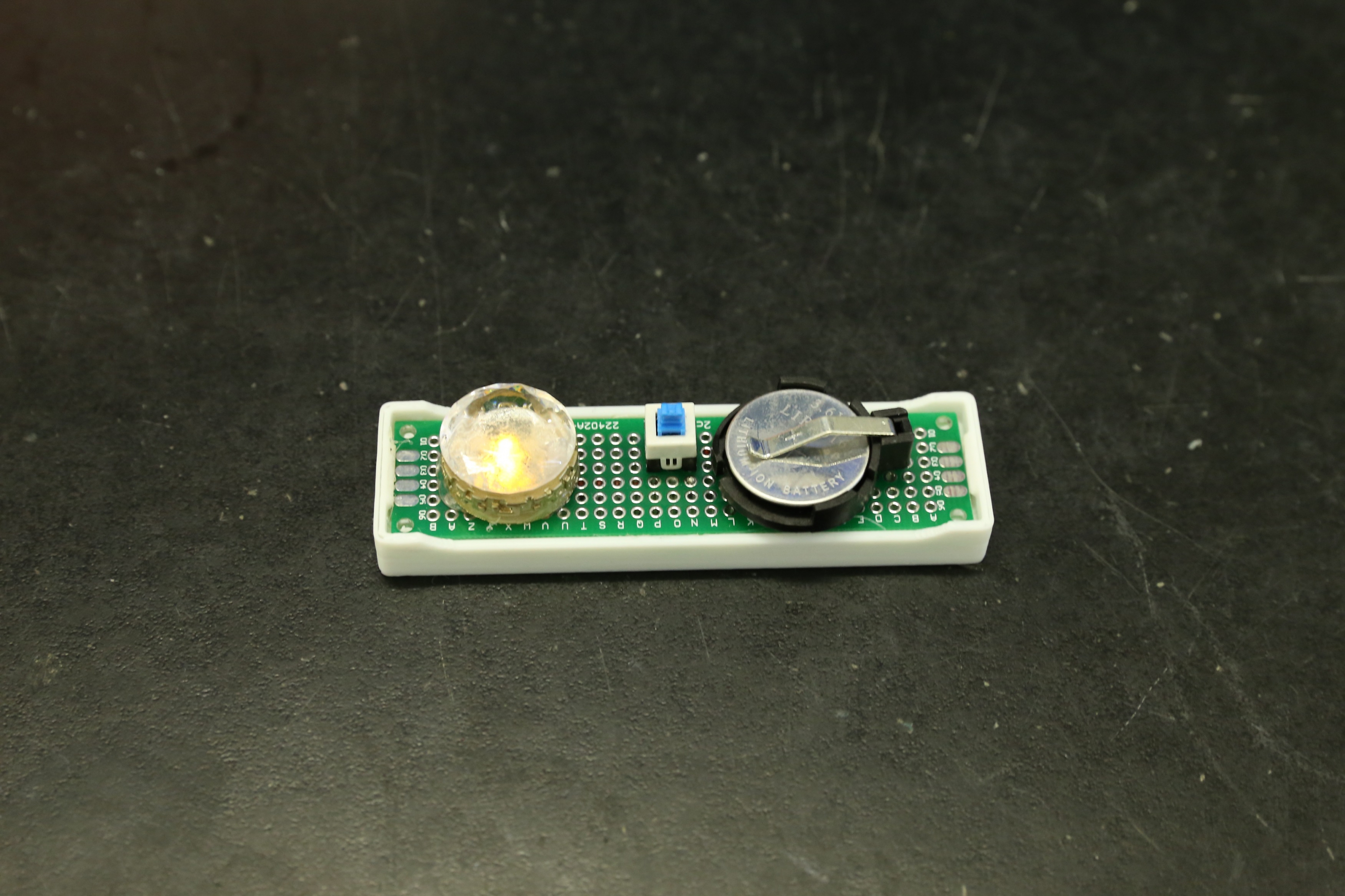
Prof. Yichun Lu and Ph.D. candidate Jing Xie present molecular crowding electrolyte and battery prototype.
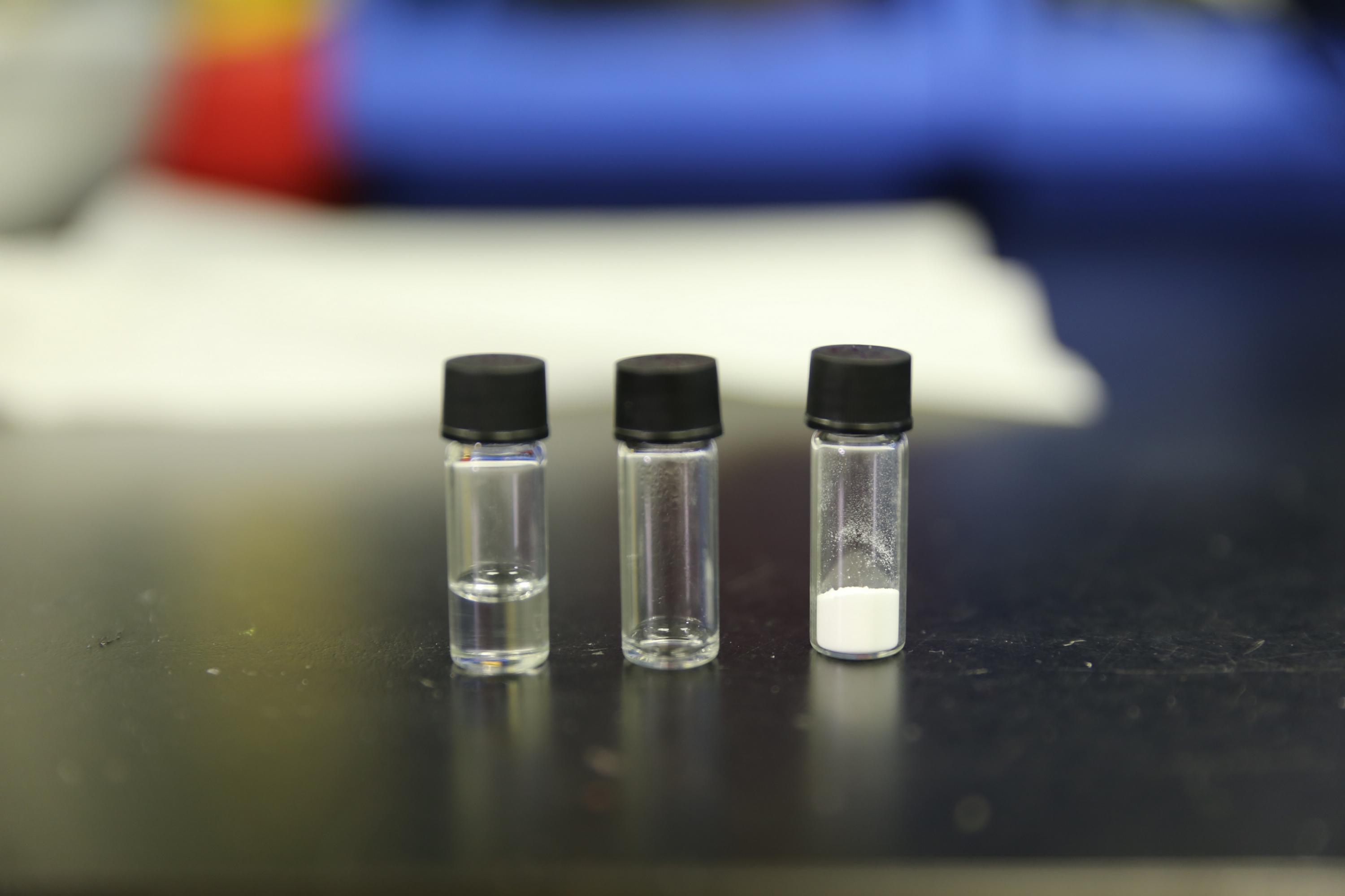
Ingredients of CUHK molecular crowding electrolyte. PEG (left), pure water (middle), lithium salt (right).
Breakthrough by CUHK Engineering in Battery ResearchElectrolyte Made with Skin Cream Ingredients Enables Stable and Non-flammable Aqueous Li-ion Batteries
A research team led by Prof. Yi-Chun LU from the Faculty of Engineering at The Chinese University of Hong Kong (CUHK) has taken a critical step forward in improving high-energy batteries by introducing a novel electrolyte to the aqueous lithium-ion (Li-ion) battery. This electrolyte is commonly used in skin cream. It is inexpensive, non-flammable, less toxic and is eco-friendly, yet can create stable voltage for common usage. The breakthrough was recently published in the world-leading scientific journal, Nature Materials, a sister journal of Nature.
The transformation of the Li-ion battery: from flammable organic to aqueous
In electronic devices and gadgets such as cell phones and laptops which have improved our daily lives on many levels, you can always find a Li-ion battery. As a result of the rechargeable characteristics and stable energy output, they have become the heart of these electronics. Despite years of research, Li-ion batteries still heavily rely on toxic and flammable organic electrolytes to produce power, and the serious safety hazards remain unsolved. The Samsung smartphone explosions and batteries on fire in a novel Boeing plane are just a few examples. These incidents have indicated that the safety of Li-ion batteries is not yet assured.
In contrast, aqueous Li-ion batteries are non-flammable and because of their water-based electrolyte, they do not pose any significant risks of explosion. However, aqueous Li-ion batteries have been suffering from low energy-density issues due to the low battery cell voltage limited by water stability. Electrolysis will occur and break down water into hydrogen and oxygen when the voltage is over 1.23 Volts, destabilising the battery operation and voltage output. Existing approaches to increase the cell voltage of aqueous batteries often involve the use of a large amount of expensive and toxic Li-ion salts to stabilise water molecules, which raises the issues of cost, toxicity and environmental sustainability.
Skin cream ingredient helps stabilise aqueous electrolyte
Building on their previous research on aqueous Li-ion batteries, Prof. LU’s team introduced “molecular crowding” into a novel aqueous electrolyte to replace Li-ion salts as the main stabilisation agent.
“Molecular crowding” is a common phenomenon in living cells, describing the fact that the properties of solution molecules can be substantially modified when macromolecules (proteins, complex sugars, polysaccharides and so on) or small hydrophilic molecules (metabolites, osmolytes) reach a certain concentration level. As a consequence of crowded environments, the activity of the water solvent is reduced owing to the changes in the water’s hydrogen-bonding structuring.
Prof. LU’s team chose to use poly(ethylene glycol) or PEG to replicate “molecular crowding” in the electrolyte. PEG is a water soluble polymer that can be easily incorporated into aqueous batteries. It is also the basis of many skin creams and personal lubricants and is even used in toothpastes and as an anti-foaming agent in food and drinks.
Using this novel stabilisation agent, a LiMn2O4 cathode and a Li4Ti5O12 anode, the team successfully expanded the aqueous electrolyte stability window to 3.2 Volts and demonstrated stable battery operation for delivering a high energy density of 75-100Wh per kg over 300 cycles using this new electrolyte; the operational voltage can be further improved to over 4.0 Volts with gel coating. The common side reactions in aqueous Li-on batteries (hydrogen / oxygen evolution reactions) are virtually eliminated. A flammability test was conducted to prove the fireproof characteristics of the novel electrolyte, significantly improving the safety of Li-ion batteries.
Prof. LU said, “This electrolyte enables the use of many electrode materials that cannot be used in the conventional aqueous electrolytes. More importantly, this research finding provides a new platform for designing an aqueous electrolyte with large-voltage window and high stability for safe, low-cost and eco-friendly energy storage.”
About Prof. Yi-Chun LU
Prof. Yi-Chun LU received her B.S. degree in Materials Science & Engineering from the National Tsing Hua University, Taiwan, in 2007. She received her Ph.D. degree in Materials Science & Engineering from the Massachusetts Institute of Technology (MIT), Cambridge, USA in 2012. Prof. LU has been a research affiliate of MIT since 2013. She is currently an Associate Professor in the Department of Mechanical and Automation Engineering at CUHK. She was awarded China’s Excellent Young Scientists Fund 2019 for her research work on electrochemical energy storage and material interface science. It is her goal to develop a stable, low-cost, scalable and rechargeable energy storage system.
Prof. LU is a Founding Member of the Young Academy of Science of Hong Kong and Associate editor of Journal of Materials Chemistry A published by the Royal Society of Chemistry. She has had conferred on her various CUHK and international research and teaching awards, including Young Researcher Award (2016), the University Education Award, CUHK (2016), the Vice-Chancellor’s Exemplary Teaching Award, CUHK (2014), the Early Career Award, Research Grant Council, Hong Kong SAR (2014), Massachusetts Institute of Technology Martin Family Society of Fellows for Sustainability (2009) and the Taiwan National Science Council Outstanding Research Innovation Award (2007).
CUHK Faculty of Engineering has produced a video to demonstrate the production of the electrolyte, fireproof and energy test of the novel aqueous Li-ion Battery. You may view it here.
Reference:
Xie, J., Liang, Z. & Lu, Y. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. (2020).
https://doi.org/10.1038/s41563-020-0667-y