CUHK
News Centre
CUHK Unveils Novel Mechanism of Neural Progenitor Competence RegulationEnlightening Path for Stem Cell Therapy and Regenerative Medicine
A research team led by Professor Kin-ming KWAN from the School of Life Sciences at The Chinese University of Hong Kong (CUHK) has recently discovered a novel mechanism by which extrinsic signaling factors modulate the fate transition of neural progenitors to allow the generation of specific neuronal subtypes. This work provides important insight into stem cell biology and the regeneration of neuronal cells, and contributes to the development of neurological therapy for diseases like autism spectrum disorders and hereditary cerebellar ataxia. The research is published in the renowned international scientific journal Cell Reports.
Some neurological diseases are related to stoichiometry of neurons
The brain, as the control centre of animals, is built up from tens of billions of neurons which can be categorized into hundreds of different subtypes, each with unique cellular characteristics and a specialised functional purpose. These neurons are differentiated from neural stem cells by undergoing temporal transition of fate specification.
During the central nervous system (CNS) development, neural stem cells sequentially express different transcription factors in accordance with a preset temporal schedule and thereby differentiate into different types of neurons. Alterations in neuronal diversities and stoichiometry are often deleterious to brain functions. The pathology of many common neurological diseases, such as autism spectrum disorders and hereditary cerebellar ataxia, are closely associated with a reduction in the number of neurons. Therefore, studying the mechanism that regulates the competence of neural progenitors is the key to developing therapeutic interventions against neurodevelopmental disorders and neurodegenerative diseases. However, how this temporal transition of neural stem cell fate is mechanistically implemented remains mysterious.
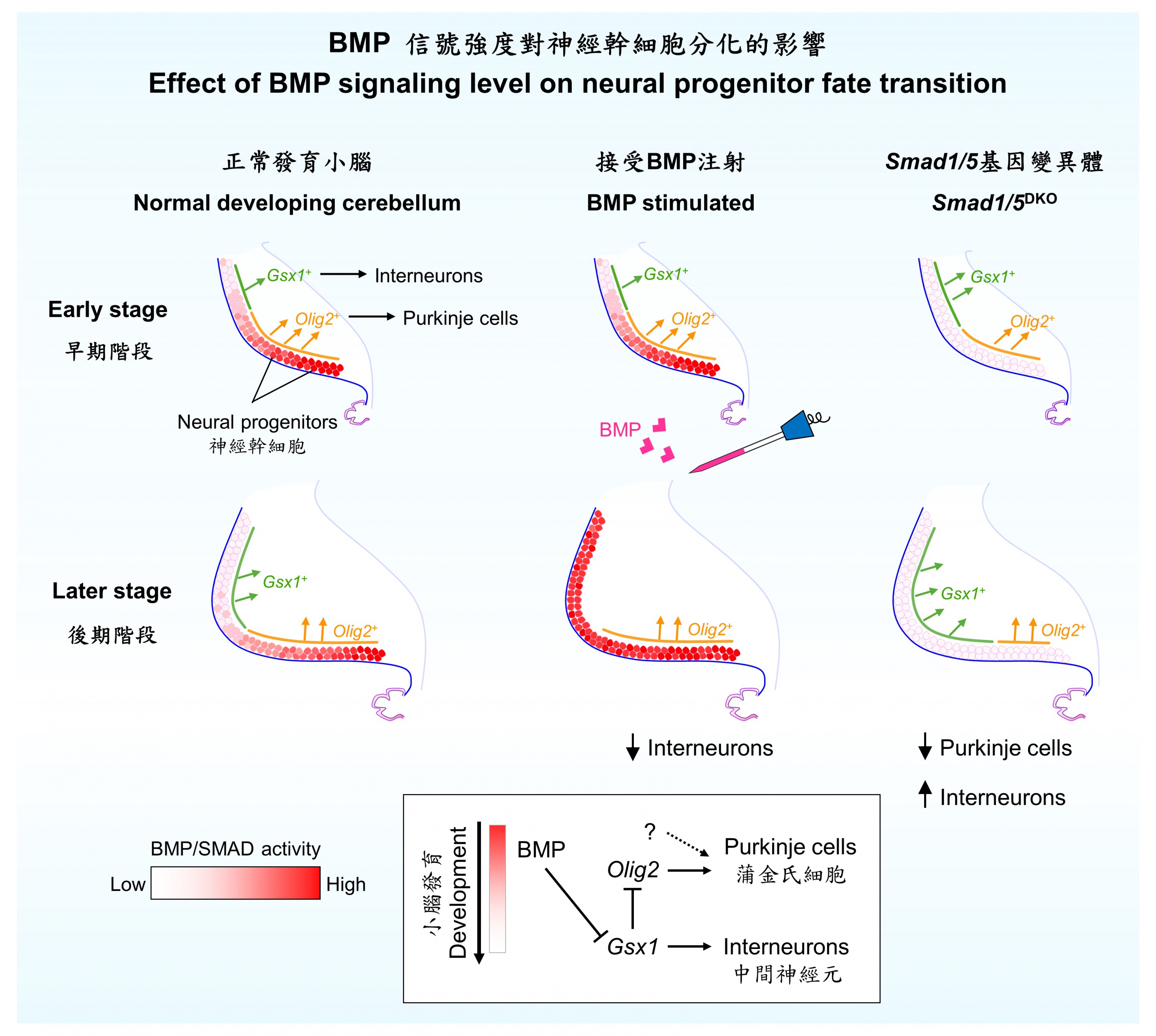
Taking the developing cerebellum as an example, neural progenitors will first differentiate into Purkinje cells. As development proceeds, progenitors gradually lose this potency and switch to generate the interneurons. Professor Kwan’s team revealed that the signaling strength of bone morphogenetic protein (BMP) determined the temporal identity transition of neural progenitors, and the gradual restriction of progenitor potency is driven by the temporal decline of BMP signaling strength.
In mutant mouse embryos lacking the two essential mediators of BMP signaling, Smad1 and Smad5, the transition of the progenitor fate specification programme is accelerated, and this consequently leads to significantly fewer Purkinje cells but remarkably more interneurons generated in the cerebellum. The team also demonstrate that SMAD1 and SMAD5 can directly repress the downstream gene Gsx1, the expression of which is a hallmark for potency restriction. Therefore, the researchers attempted to inject recombinant BMP signaling molecules into the cerebrospinal fluid of mouse embryos, causing a sharp increase of BMP signaling strength in the progenitor pool of the developing cerebellum. Interestingly, this approach is able to stall the restriction of stem cell competence and alter the stoichiometry of specific neuronal subtypes.
“The generation of distinct neuronal subtypes from neural progenitors requires a change of progenitor competence. However, what drives this process has been a central question in the field. Our research for the first time shows that in mammalian brains, the strength of extrinsic signaling factors plays a decisive role in instructing the expression of transcription factors to regulate progenitor fate transition,” Professor Kwan explained, “By simply adjusting the strength of certain extrinsic signaling cues, it is already possible to modulate the competence of neural progenitors. This finding is implying immense potential in engineering neural progenitors for therapeutic purposes through the manipulation of extrinsic cues, and we are taking a further step to assess this feasibility.”
This project was supported by the General Research Fund and Areas of Excellence (AoE) Scheme of the Hong Kong Research Grants Council, as well as the Centre for Cell and Developmental Biology and State Key Laboratory of Agrobiotechnology of CUHK.
Brief biography of Prof. Kin-ming Kwan
Prof. Kin-ming Kwan is Associate Dean (Education), Associate Professor and Director of the Natural Sciences Programme in the Faculty of Science at CUHK. He received undergraduate and doctoral training at The University of Hong Kong, and postdoctoral training at The University of Texas MD Anderson Cancer Center (US). Since 2006, Professor Kwan has been investigating the genetic regulation of neuronal cell development in cerebellum and its relationship to stem cell renewal and neuronal cell regeneration. He joined CUHK in 2006 and is the recipient of the CUHK Young Researcher Award, 2008 and the CUHK Science Faculty Teaching Award, 2009 and 2013.
Note:
Spatiotemporal Decline of BMP Signaling Activity in Neural Progenitors Mediates Fate Transition and Safeguards Neurogenesis, Cell Reports
https://www.cell.com/cell-reports/fulltext/S2211-1247(20)30263-1
Professor Kwan (right) and his research team unveils the mechanism of neural progenitor competence regulation