CUHK
News Centre
CUHK Team develops a big data analysis method to study senescent neurons
Providing new directions for precision medicine in neurodegenerative diseases
A research team led by Professor Kim Chow Hei-man in The Chinese University of Hong Kong (CUHK)’s School of Life Sciences has developed a data-driven bioinformatics analysis workflow to facilitate the identification of distinct features and biomarkers of senescent neurons in normal ageing and demented brains. The research team revealed that mature neurons that recommit into a cell cycle-like senescent process have a significant impact on the onset and progression of Alzheimer’s disease and related forms of dementia. Findings of this study have been published in the prestigious international journal PLOS Biology, which selected it as a feature article.
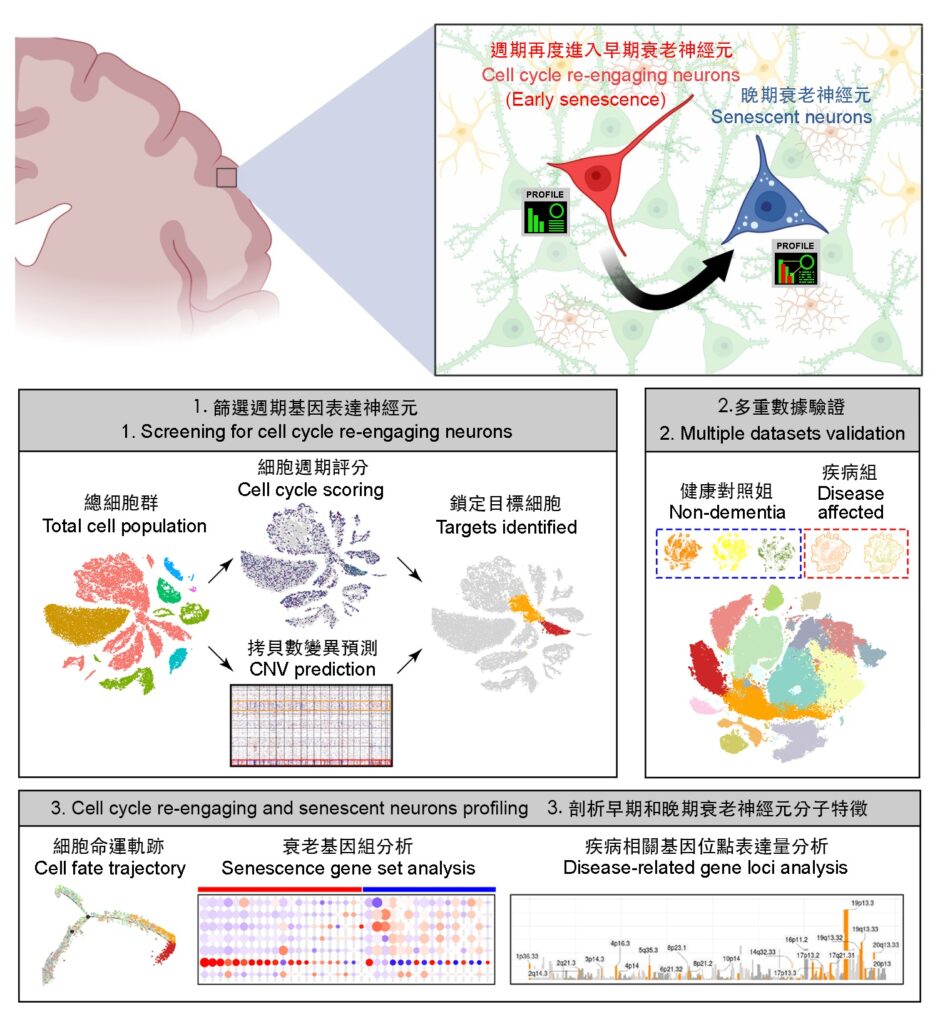
The cell cycle refers to the continuous process of cell division, growth and subsequent division. During this continual process, cells eventually become old until they are replaced by newly generated cells. Unlike typical cells, fully differentiated neurons in the brain cease to divide and themselves fail to regenerate once they become fully mature. According to previous studies published by the same research team, certain neuronal populations in the brain may recommit into a cell cycle-driven senescent process under various stress conditions. However, due to the limitations of the traditional research methods, it has been challenging to predict the molecular changes in these cells accurately and objectively.
New study approach overcomes hurdles
Due to the unpredictable distribution and relatively low density of these cell cycle re-engaging and senescing neurons emerging throughout the brain, traditional histology and bulk tissue transcriptomic techniques are unable to identify the molecular characteristics and development processes, as well as their potential impact on the neighbouring cells. Furthermore, conventional experimental methods are unable to distinguish whether these ageing neurons exhibit any disease specificity due to the diverse microenvironment of the brain.
The research team has developed a customised bioinformatics analysis workflow based on the unique biological properties of neurons, which also enables integrated analyses of multiple brain single-nucleus transcriptomic data. The team tested this approach with multiple sets of transcriptomic data from the brains of individuals with Alzheimer’s disease in different countries, along with the clinical data of these patients. With the new study approach, they identified how the senescing neurons are characterised by specific markers of different stages of the cell cycle, senescent signatures and functional changes. The team reconfirmed that neurons with reactivated cell cycle machinery do not complete the cell division process to regenerate new progeny neurons; rather, they undergo accelerated ageing and accumulate aberrantly to affect the overall brain function and homeostasis.
Accumulations of senescent neurons are related to advanced stages of Alzheimer’s disease
According to Professor Chow, “The total number of these cell cycle re-engaging and senescing neurons is positively correlated to more advanced stages of Alzheimer’s disease, and their accumulation is related to more severe pathologies of the disease, with aberrant expression of known disease risk genes. Similarly, accumulations of these abnormal neurons were identified, as well in the case of Parkinson’s disease-Lewy body dementia. This new study approach allows researchers to gain a deeper understanding of the intrinsic properties of these cells and their uniqueness when diseased, which may assist the development of precision medicine.”
Using Alzheimer’s disease as the primary study model, the analyses revealed that cell cycle re-engaging and senescing neurons are functionally defective compared to healthy neighbouring neurons. In the healthy ageing brain, the numbers of these neurons undergoing early- and late-stage ageing are regulated under normal brain tissue homeostasis. However, in Alzheimer’s disease, these cells accumulate instead. Moreover, they are characterised by a unique set of biomarkers that known to promote pro-inflammatory responses, metabolic dysfunction and pathology-related molecular characteristics.
The research team also extended their analysis to investigate senescent neurons in a model of Parkinson’s disease-Lewy body dementia. Additionally, they conducted an extensive analysis of brain ageing using a mouse model, which further validated the efficacy of their bioinformatics approach in characterising the profiles of senescent neurons across different species. The demonstrated applicability of this analytical approach in diverse disease models and cross-species settings opens new opportunities and insights that complement traditional histological-based approaches in studying the roles of senescent neurons in brain ageing and disease pathogenesis. The ability to uncover disease-specific molecular signatures and identify novel marker genes in senescent neurons may also pave the way for new directions in future diagnostics and the development of senotherapeutic strategies.
Other important contributors to the study include Dr Wu Deng and Miss Jacquelyne Sun Ka-li, Postdoctoral Associate and final year PhD student at the CUHK School of Life Sciences.