CUHK
News Centre
CUHK Reveals a Novel Mechanism to Stimulate Neurite OutgrowthPaving a New Road for Brain Regenerative Medicine
A team of scientists led by Professor Kwok-Fai LAU, Associate Professor of the School of Life Sciences at The Chinese University of Hong Kong (CUHK), has recently discovered a novel mechanism that stimulates a process called neurite outgrowth – the growth of nerve cell (neuron) projection. This finding provides important insights into developing strategies to stimulate neurite regeneration after nerve injury caused by traumatic brain injury (TBI) and in neurodegenerative disorders. This research is published in the May issue of the Journal of Biological Chemistry, the prestigious journal of the American Society for Biochemistry and Molecular Biology.
TBI occurs when an external force injures the brain. It usually results from falls, car accidents, sports-related injuries and beatings. Severe situations may lead to permanent disability. Neurodegenerative disorders are symptoms of loss of function in brain and spinal cord cells, including Alzheimer’s disease, Parkinson’s disease, spinocerebellar ataxia, and amyotrophic lateral sclerosis. About 5% to 8% of elderly people in Hong Kong suffer from dementias, most of whom have Alzheimer’s disease, placing a heavy burden on society. In these diseases, a damaged neural network is observed, in which degeneration and retraction of neurite are found.
The brain is the command centre of animals and is composed of neurons interconnected by neurite, grown out from their cell bodies. Such connections are essential for the formation of neural networks which allow the communication of neurons to regulate different cognitive functions and body activities. However, when neurites are degenerated and retracted, the connections of the neural network cannot be maintained and the cognitive and body’s motor functions will be difficult to recover. At present, there is no cure for nerve damage. CUHK School of Life Sciences has discovered a mechanism that stimulates neurite outgrowth. As long as two specific proteins are introduced into the neuron, their interactions can increase the length of neurites by at least two times and bring new hope for the reconnection of impaired neural networks.
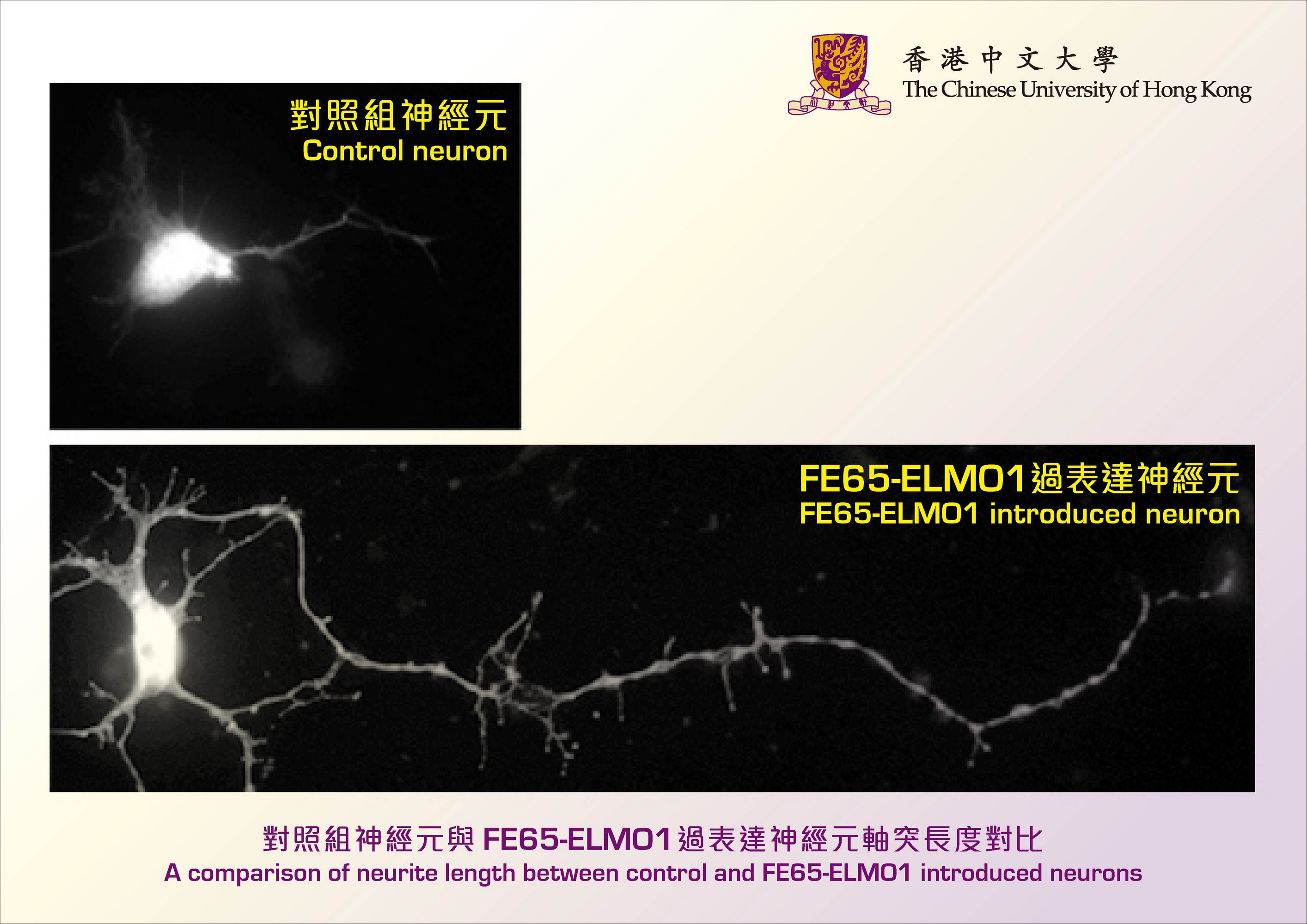
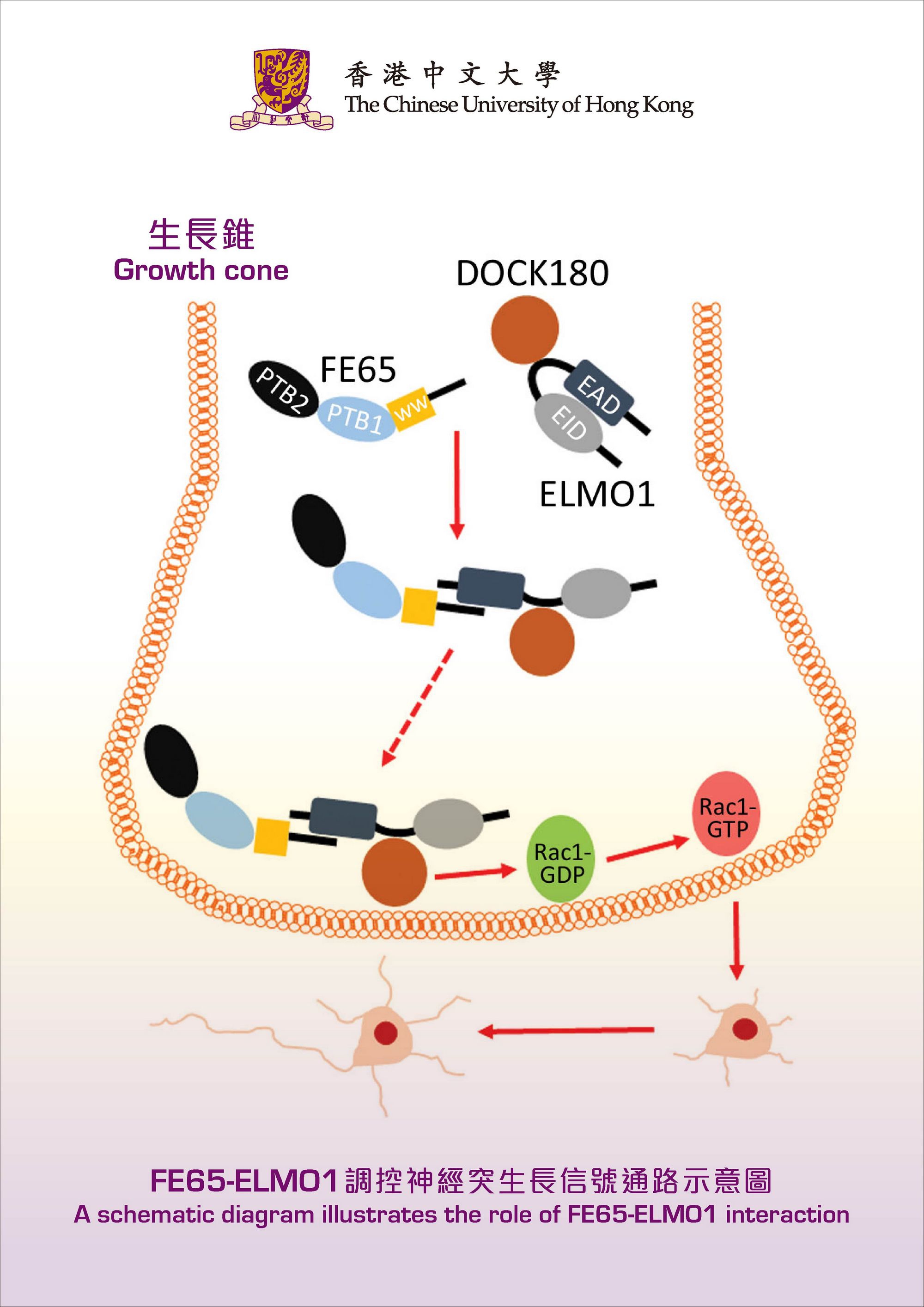
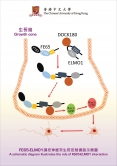
Professor Lau’s team has found that the interaction between two proteins, named FE65 and ELMO1, strongly stimulates neurite outgrowth. FE65 is a brain-enriched adaptor that is implicated in nervous system development, while ELMO1 is a widely expressed protein that participates in various processes including cell migration. However, the role of ELMO1 in the nervous system has never been reported. By introducing FE65 and ELMO1 to mammalian neurons, the length of neurite was increased by at least two-fold. Conversely, such stimulatory effect was not observed when the interaction was interrupted. The team further demonstrated that such interaction promotes the transport of ELMO1 to the plasma membrane where it activates Rac1, a key regulator of cytoskeleton, the remodeling of which is required for neurite extension.
One major obstacle in treating neurodegenerative disorders, including Alzheimer’s disease, is how to re-connect the neurons in the brain of the patients. Professor Lau believes that their work has provided a new direction in regenerative medicine for the injured brain. He said, ‘Re-connection of injured neurons could be achieved by the stimulation of neurite re-outgrowth in these cells through manipulating FE65-ELMO1 interaction.’ Most recently, the team has obtained new data regarding how to regulate the interaction.
Biography of Professor Kwok-Fai Lau
Professor Kwok-Fai Lau obtained his BSc degree in biology at the Hong Kong Baptist University. Thereafter, he received a PhD degree in biochemistry at CUHK. In 1999, he was awarded a Croucher Foundation fellowship and relocated to the Institute of Psychiatry in London UK where he started his work on Alzheimer’s disease. In 2007, he returned to his alma mater as an Assistant Professor in the Department of Biochemistry. Currently, he is an Associate Professor in the School of Life Sciences. Since he returned to CUHK, he has published his work in several leading international journals including Nature Communications, PNAS, FASEB Journal and the Journal of Biological Chemistry. He received the Science Faculty Exemplary Teaching Award in 2014.
(From left) Professor Edwin Chan, Professor Kwok-fai Lau and Professor Jacky Ngo from the School of Life Sciences, CUHK.
Professor Kwok-fai Lau, School of Life Sciences, CUHK (front) and his team members (from left, back row): Professor Jacky Ngo, Professor Alex Koon, Professor Edwin Chan, Mr. Ray Chan, and Dr. Wen Li.