CUHK
News Centre
CUHK leading Asia’s battle to improve bile duct cancer survival rates by developing a novel combinational strategy in clinical trial
A multicentre clinical trial led by The Chinese University of Hong Kong’s (CUHK) Faculty of Medicine (CU Medicine) with overseas medical institutions showed that the combination of selective internal radiotherapy (SIRT) and conventional chemotherapy is effective in treating intrahepatic cholangiocarcinoma (ICC) that is not suitable for surgery.
The standard first-line treatment for inoperable ICC is chemotherapy, which is associated with a median overall survival of 11.7 months. With the use of SIRT before chemotherapy, this clinical trial found that the median overall survival of patients with inoperable ICC could be improved to 21.6 months among those undergoing both treatments. The results were published in the renowned journal Liver Cancer.
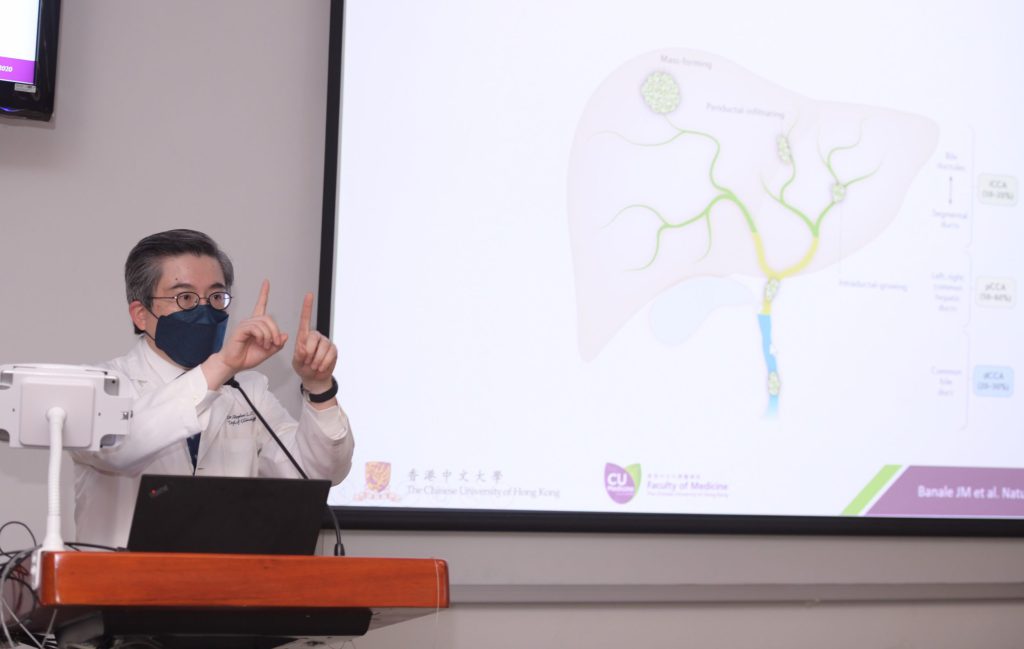
Intra-hepatic cholangiocarcinoma is a growing health concern internationally, with increasing incidence
Biliary tract cancer is traditionally considered a less common cancer, accounting for only 3% of digestive tract cancers. However, the incidence of ICC, a subtype of biliary tract cancer arising from the bile duct in the liver, has been increasing over the past decade due to obesity and unhealthy lifestyles.
Over 80% of patients with ICC suffer from locally advanced or metastatic disease, rendering them unsuitable for surgery. These patients frequently present with symptoms such as jaundice, abdominal pain or weight loss. Researchers around the world are working hard discover better treatments for this group of patients.
A potential new option for patients with inoperable ICC
The clinical trial was designed and initiated by CU Medicine’s Department of Clinical Oncology, in collaboration with the Department of Imaging and Interventional Radiology, and with the subsequent participation of three more sites in Asia, namely the Chulabhorn hospital in Thailand, the National Cancer Centre Singapore, and the National University of Singapore’s Cancer Science Institute.
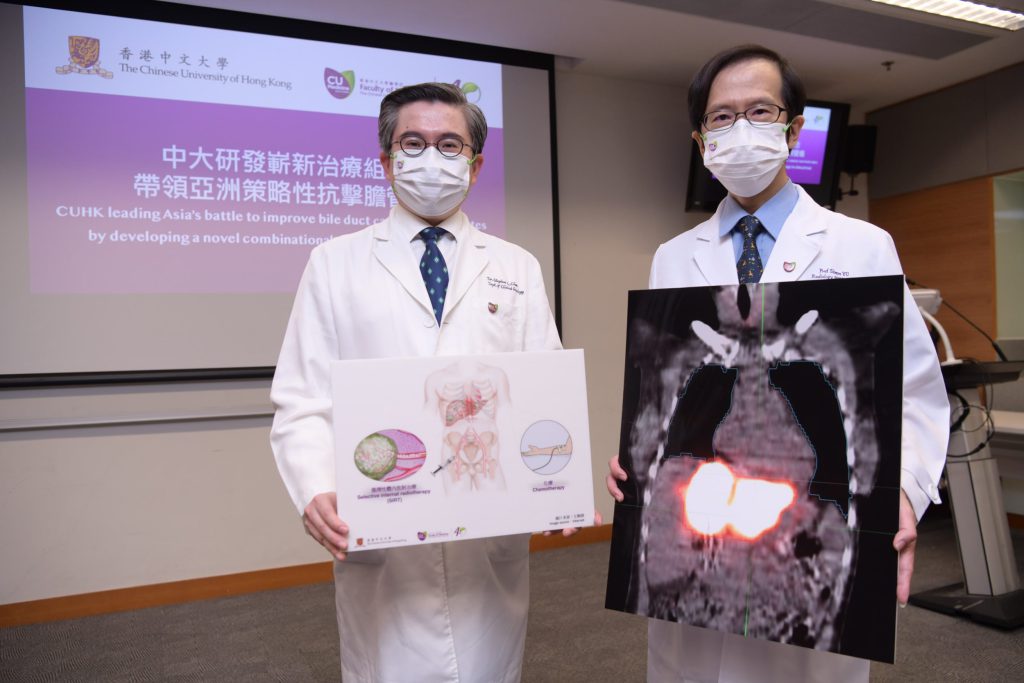
31 patients were screened, of whom 24 were recruited for the trial. The participating patients underwent screening for treatment by SIRT with yttrium 90, a regional therapy for tumour in the liver, followed by standard chemotherapy. CU Medicine’s Department of Imaging and Interventional Radiology Professor Simon Yu Chun-ho, highlighted the role of regional therapy in ICC. “SIRT involves the trans-arterial injection of radioactive materials containing yttrium-90 into the liver tumour. Although its role has been proven in hepatocellular carcinoma, there is a lack of clinical trial data on the role of SIRT in ICC, especially in the context of a standard chemotherapy regimen,” he said.
The results showed that the median overall survival of patients who underwent SIRT, regardless of chemotherapy, was 13.6 months; and that for the group of patients who underwent both SIRT and chemotherapy, it was 21.6 months. Among 16 patients of the trial group, 25% experienced shrinkage of tumour and 75% had the disease under control. The new treatment was generally safe, with fewer than 10% of patients developing moderate to severe side effects.
“Conducting an investigator-initiated, multicentre clinical trial in a less common cancer is challenging but extremely meaningful,” said Professor Stephen Chan Lam, Ip’s Family Trust Professor of Clinical Oncology of the Department of Clinical Oncology at CU Medicine and the trial’s principal investigator.
“Generally, the pharmaceutical industry is less interested in exploring treatment for less common cancers. It is the responsibility of clinical researchers at CUHK to look for better treatments for patients. I am grateful for the efforts of the four centres in three different regions to address this unmet need of our patients. Our data will give a potential option for more intensive treatment in selected patients, where the main disease burden is in the liver,” Professor Chan added.
A multicentre clinical trial led by CU Medicine with overseas medical institutions showed that the combination of selective internal radiotherapy (SIRT) and conventional chemotherapy is effective in treating intrahepatic cholangiocarcinoma (ICC) that is not suitable for surgery. The median of the overall survival of patients treated with the novel combination strategy is 80% more than those treated with chemotherapy alone.
(From left) Professor Stephen Chan, Ip’s Family Trust Professor of Clinical Oncology of the Department of Clinical Oncology at CU Medicine; and CU Medicine’s Department of Imaging and Interventional Radiology Professor Simon Yu Chun-ho.