CUHK
News Centre
CUHK-HKU-UCL study unravels how gene mutation leads to congenital megacolon providing clues for the development of novel therapeutic strategies
Hirschsprung’s Disease (HSCR), or congenital megacolon, is a common congenital gastrointestinal motility disorder in newborn babies. Patients with HSCR may carry different types of gene mutation. To understand the pathogenesis of this disease, a collaborative study by researchers from The Chinese University of Hong Kong (CUHK), The University of Hong Kong (HKU) and University College London (UCL) Great Ormond Street Institute of Child Health in the UK has revealed that mutation in the Sox10 gene retarded the migration of neural progenitor or stem cells which led to a deficiency in neural cells in the gut, resulting in gut motility or movement problem.
The team further showed that Sox10 gene mutation is linked with the reduction of cadherin-19, a molecule that is essential in regulating the early development of gut neural cells. Hence, supplementation of cadherin-19 could be a potential therapy for HSCR patients. These findings have been published in the international journal Gastroenterology.
Surgery cannot fully cure the congenital megacolon and more treatment options are needed
One in every 5,000 live newborns in the world are suffering from HSCR. This disease is even more common among Chinese, with an incidence of 1 in 3,500 newborn babies. In HSCR patients, a varying length of the gastrointestinal tract does not stretch or contract normally (i.e. gut motility or movement disorder) and affected babies show defecation difficulties immediately after the birth, leading to the accumulation of intestinal content and enlargement of the colon. In severe cases, the babies may have swollen abdomens. If left untreated, the disease can be life-threatening.
Professor Paul Kwong Hang TAM, Chair Professor of Paediatric Surgery, Department of Surgery from LKS Faculty of Medicine, HKU, stated, “Babies with HSCR are required to undergo surgical removal of the affected segment of the colon and reconnection of the normal colonic parts. Unfortunately, even after the surgery, many babies still show gastrointestinal problems such as faecal incontinence and intestinal infection which may last for a long time or even throughout the rest of their lives.”
Study proves Sox10 gene mutation can retard development of neural cells in the gut
The collaborative research team has been investigating the Neural Crest Cells (NCC), a special type of neural progenitor cells which migrate to the gut to form neural cells during early embryonic development. In an animal model of HSCR, if the migration of NCC is disturbed, the number of neural progenitor cells that reach the gut will be reduced, resulting in a reduction of the number of neural cells that are ultimately formed in the gut. As these neural cells regulate peristalsis or movements of the gut, the deficiency in neural cells leads to motility problems.
In this study, the researchers found that the Sox10 mutation retarded the migration of NCC to the developing gut, leading to a reduction of neural progenitor cells in the gut. In addition, the team found that Sox10 mutant was unable to bind to the gene for producing cadherin-19, resulting in the decrease in cadherin-19 level. Using an animal model of HSCR with Sox10 gene mutation, cell migration was found to be retarded when cadherin-19 level was low, whereas normal cell migration was restored when cadherin-19 supplemented the defective neural progenitor cells.
Professor Woody Wood Yee CHAN, Professor of the School of Biomedical Sciences, CU Medicine and the corresponding author of the publication, said, “Our new findings not only unravel the mechanism of how Sox10 mutation leads to HSCR, but also suggest that cadherin-19 could be a potential therapeutic target for HSCR. Therapeutic strategies could be developed to increase the level of cadherin-19 in the gut to help rescue defective cell migration in HSCR patients.”
Professor Mai Har SHAM, Pro-Vice-Chancellor of CUHK and Professor of the School of Biomedical Sciences, CU Medicine, stated, “Our study revealed how a genetic mutation could lead to problems with cell-cell interactions and cell movements, expanding our scope for addressing disease mechanisms and treatment options. We are grateful for the collaboration in the process of discovery and innovation.”
This project was supported by grants from the Theme-based Research Scheme (TRS) and the General Research Fund (GRF) of Hong Kong Research Grants Council (RGC).

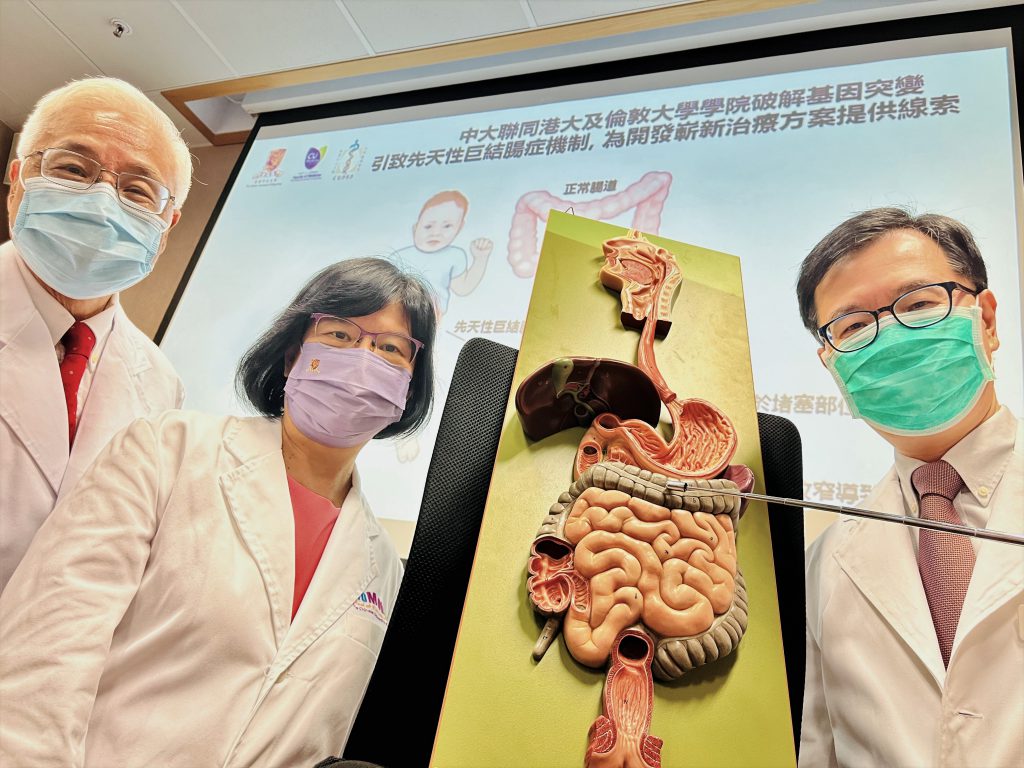
A collaborative study by Prof. Mai Har SHAM (centre), Pro-Vice-Chancellor of CUHK; Prof. Woody CHAN (right), Professor of the School of Biomedical Sciences from CU Medicine; and Prof. Paul TAM (left), Chair Professor of Paediatric Surgery from HKU LKS Faculty of Medicine, and UCL Great Ormond Street Institute of Child Health in the UK, has revealed that Sox10 gene mutation in patients with congenital megacolon is linked with the reduction of cadherin-19. It is a molecule that is essential in regulating the early development of gut neural cells, thus supplementation of cadherin-19 could be a potential therapy for those patients.