CUHK
News Centre
CUHK-HKU collaborative research finds a new inflammatory activation pathway in blood vessels after SARS-CoV-2 infection
A study led by The Chinese University of Hong Kong’s (CUHK) Faculty of Medicine (CU Medicine) and the LKS Faculty of Medicine, The University of Hong Kong (HKUMed) provides new insight into how coronavirus SARS-CoV-2 triggers severe inflammation in human blood vessels. They discovered that SARS-CoV-2 can induce endothelial inflammation through activation of a unique cell surface receptor, toll-like receptor (TLR) 4, without entering the host cell. The findings have been published in Stem Cell Reports.
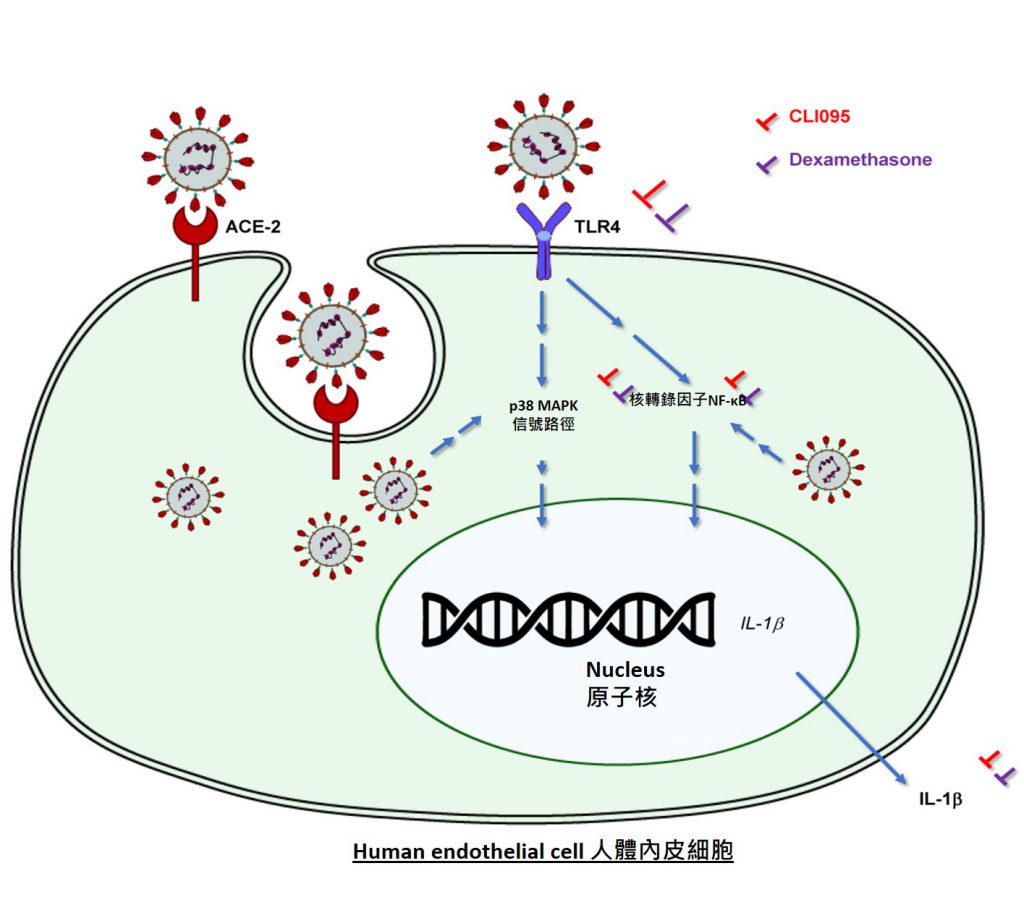
In the search for medications for COVID-19, scientists have given much attention to a specific protein, receptor ACE2, which provides an entry point for SARS-CoV-2 to infect human endothelial cells, the innermost layer of a blood vessel. This process has been generally acknowledged to trigger hyperinflammation, blood clotting and thrombosis in severe COVID-19 patients. However, researchers from CU Medicine and HKUMed provide a contrary view after carefully studying the expression of ACE2. They found that adult and fetal endothelial cells derived from humans rarely express ACE2 on the cell surface, indicating that viral entry through ACE2 may not be the main cause of endothelial inflammation.
Study uses stem cells to generate endothelial cells for modeling COVID-19
During the investigation, one of the concerns about the ACE2 finding is that the commercially available cell sources are not freshly derived and have been cultured for some time already, which could change their characterisation. Therefore, the research team immediately generated endothelial cells from human pluripotent stem cells that are capable of making almost all kinds of human cells, including endothelial cells. They then infected these cells with live SARS-CoV-2 extracted from COVID-19 patients for further study.
Professor Kathy Oi Lan LUI, Associate Professor of the Department of Chemical Pathology at CU Medicine said, “By virtue of their capability of self-renewal, we can generate unlimited numbers of endothelial cells from human pluripotent stem cells for this study, and avoid invasive procedures that only allow us to get limited numbers of these cells from patients.”
SARS-CoV-2 can directly induce endothelial inflammation through activation of receptor TLR4
Although one may think that the expression of ACE2 facilitating viral entry could be harmful, research findings demonstrated that it also protects human endothelial cells from hyperinflammation after SARS-CoV-2 infection. Moreover, SARS-CoV-2 can directly induce endothelial inflammation through activation of another cell surface receptor TLR4, without host cell entry. TLR4 is an innate immune receptor expressed on myeloid immune cell surface that is best-known for recognising lipopolysaccharide (LPS) derived from bacteria. The study shows that endothelial TLR4 can recognise SARS-CoV-2, contributing to vascular inflammation that can be blocked by TLR4-specific inhibitors.
Circulating endothelial cells of mild and severe COVID-19 patients display activated gene signatures of the TLR4 signaling pathway
Furthermore, by analysing the genome of circulating endothelial cells collected from mild and severe COVID-19 patients at a single-cell resolution, the study further reveals that circulating endothelial cells of these patients display activated gene signatures of the TLR4 signaling pathway.
Professor Leo Lit Man POON, Professor and Head of Division of Public Health Laboratory Sciences, School of Public Health, HKUMed commented, “Our findings now open the door for more studies to validate whether these genes could serve as biomarkers for diagnosis of severe COVID-19 cases particularly those with the risk of fatal thromboembolism.”
This is a proof-of-concept research that demonstrates the potential of using human pluripotent stem cells for modeling SARS-CoV-2 infection in blood vessels. Professor Lui added, “The human stem cell platform can also be applied to other organ systems for modeling COVID-19, and be operated in a patient-specific manner if we collect skin fibroblasts from COVID-19 patients to generate the induced pluripotent stem cells, tailoring personalised medicine, given that disease severity varies among individuals.”

A study led by CU Medicine and HKUMed provides new insight into how coronavirus SARS-CoV-2 triggers severe inflammation in human blood vessels. They discovered that SARS-CoV-2 can induce endothelial inflammation through activation of a unique cell surface receptor, toll-like receptor (TLR) 4, without entering the host cell.
Principal Investigators of the study include Professor Kathy LUI, Associate Professor of the Department of Chemical Pathology at CU Medicine and Professor Leo POON, Professor and Head of Division of Public Health Laboratory Sciences, School of Public Health, HKUMed.