CUHK
News Centre
CUHK Engineering develops energy-efficient redox flow battery with biomimetic molecular catalysts
- As 100 countries committed at COP28 to tripling global renewable energy use by 2030, the demand for large-scale energy storage is set to increase sharply. Sulphur-based redox flow batteries may be the best solution but their low energy efficiency makes them difficult to commercialise.
- A CUHK professor has proposed a molecular catalyst that can energise the flow battery with a fast reaction rate, decreasing the overpotential for more than three times and increasing the charging power by nearly six times.
A research team led by Professor Yi-Chun Lu, Professor in the Department of Mechanical and Automation Engineering at the Faculty of Engineering at The Chinese University of Hong Kong (CUHK), has successfully developed a biomimetic molecular catalyst to enable a low-cost, energy-efficient, sulphur-based redox flow battery via homogeneous catalysis, successfully tackling the bottleneck of the poor kinetics of sulphur-based redox flow batteries. A report on the breakthrough was recently the cover story of world-leading scientific journal Nature Energy’s December issue.
The application of conventional sulphur-based redox flow batteries is limited by poor kinetics and low energy efficiency
At the United Nations Climate Change Conference (COP28), around 100 countries pledged to treble global renewable energy use by 2030, which is greatly increasing the demand for large-scale energy storage. Although lithium-ion batteries are commonly used to store energy, there is a risk of combustion and explosion when thousands of them are stacked together, making them unsuitable at large scales. Aqueous redox flow batteries are one of the most competitive technologies for large-scale energy storage due to their high safety, low cost and design flexibility in power and energy. However, further development of conventional commercial redox flow batteries is hindered by the high cost of vanadium, the active material.
Sulphur-based redox flow batteries are extremely attractive because sulphur is abundant, meaning its price is 1,000 times lower than vanadium. However, there are bottlenecks in the commercialisation of sulphur-based redox flow batteries: poor cycle life and low energy efficiency. In 2021, Professor Lu and her team proposed a charge-reinforced ion-selective (CRIS) membrane to significantly improve the cycle life by reducing the crossover rate of polysulphide, leaving the low energy efficiency as the major bottleneck for practical commercialisation.
Molecular catalysts as ‘battery vitamin’ to enable energy-efficient redox flow batteries
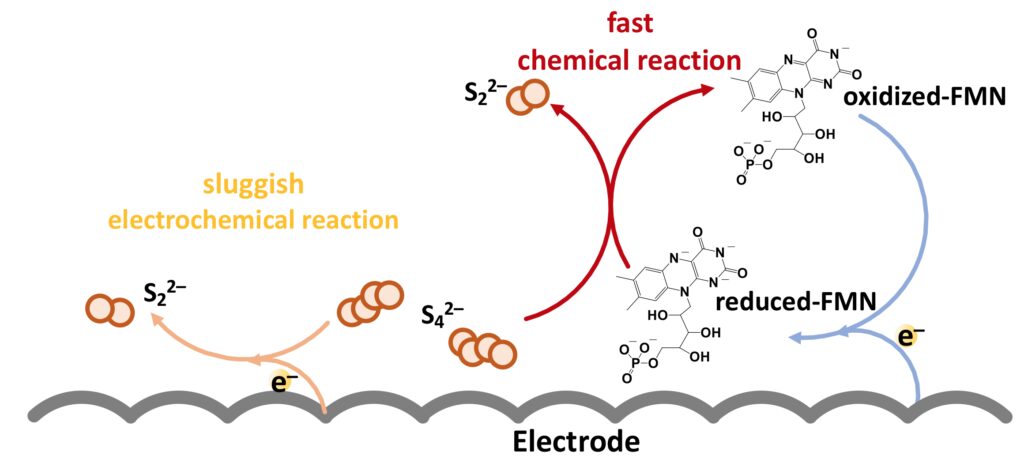
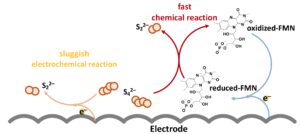
Professor Lu and her team proposed a molecular catalyst, riboflavin sodium phosphate (FMN-Na), to accelerate the polysulphide conversion with high energy efficiency. Riboflavin, known as vitamin B2, is a common vitamin that can be found in food and dietary supplements. In the human body, riboflavin derivatives function as an energy carrier. Inspired by the natural electron transfer chain, Professor Lu and her team adopted FMN-Na to energise the flow battery with a fast reaction rate.
This biomimetic strategy could dramatically decrease the overpotential by more than three times and increase the energy efficiency from 53% to up to 76%, meaning the charging power increases by nearly six times. The iron-sulphur flow batteries operated stably for over 2,000 cycles (projected lifetime > 20 years) and this facile strategy was also applied to sulphur-iodide flow batteries with high stability for over 1,300 cycles. Professor Lu and her team cooperated with Luquos Energy, an energy storage start-up founded by Professor Lu, to demonstrate a 100 cm2 cell stack with a high operating current density of 100 mA cm–2, which has great potential for practical application.
Professor Lu said, “Our work shows that homogeneous catalysis is an effective approach to addressing the sluggish kinetics of polysulphide. We believe that this approach can be widely applied to other flow batteries systems.”
The full text from the journal can be found at:

Professor Lu Yi-chun (right) and the first author Lei Jiafeng (left), a PhD student in CUHK’s Department of Mechanical and Automation Engineering, show the prototype of the sulphur-iron redox flow battery with the molecular catalyst.