CUHK
News Centre
CUHK devises new CO2 recycling electrolyser as part of journey towards carbon neutrality
A research team led by Professor Ying WANG, Assistant Professor from the Department of Chemistry at The Chinese University of Hong Kong (CUHK), has developed an electrochemical system, acidic CO2 electrolyser, to convert carbon dioxide (CO2) and water (H2O) with renewable electricity into valuable chemical raw materials, providing a promising low-carbon alternative for fossil fuel-based materials in chemical industries. The work has achieved a CO2 utilisation efficiency as high as 60% in the conversion process. The result has been recently published in Nature Catalysis, one of the world’s leading scientific journals.
Low carbon utilisation is the biggest limitation of current CO2 electrolyser technology
Mitigating emissions of greenhouse gases is an important way to combat global warming, but cutting the concentration of atmospheric carbon dioxide is of equal importance. Close to 80% of overall CO2 emissions on Earth come from the combustion of fossil fuels for chemosynthesised raw materials valuable to compositing cosmetics, detergents, plastics and more. Carbon capture and conversion technology like CO2 reduction reaction (CO2RR) is therefore among the most promising means to reduce reliance on fossil fuels, capturing CO2 emissions from industrial processes and converting the CO2 into raw materials for the production of these chemical products.
At present, electrochemical CO2RR usually operates in alkaline reactors or electrolytes, during which more than 85% of CO2 is consumed by the electrolyte, leading to the formation of carbonate, requiring extensive energy and cost to further capture and convert CO2, and resulting in a low carbon utilisation rate of 25% and poor cost effectiveness.
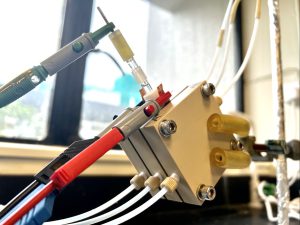
Novel acidic CO2 electrolyser with palladium-copper catalyst boosts carbon utilisation efficiency
To overcome the low carbon utilisation rate and the loss of CO2 during conversion, Professor Wang worked with a research team from the Department of Electrical and Computer Engineering, University of Toronto to develop a novel acidic CO2 electrolyser that effectively suppresses carbonate formation. The team synthesised palladium-copper (Pd-Cu) catalyst to further suppress the issue of hydrogen evolution reaction (HER) brought by an acidic condition, and successfully raised the carbon utilisation rate to 60%, under the industrial condition of current density at 500 mA/cm2. This allows more captured CO2 to be converted to valuable raw materials needed for synthesised chemicals, such as ethylene.
Professor Wang said, “The rational design of catalyst and reactor in this work breaks the theoretical limit of carbon utilisation efficiency in current alkaline CO2RR electrolysers, and contributes to the development of environmental technology. The leap in the conversion rate of CO2 in acidic electrolysers advances this green technology towards an economically viable future. Now we are working on scaling up the technology to commercialise it.”
The full research paper can be found at: https://www.nature.com/articles/s41929-022-00788-1
About Professor Ying WANG
Professor Wang joined CUHK in 2019. Her research group focuses on understanding and device engineering for electrocatalysis. This project was funded by the Early Career Scheme under the Research Grant Council.