CUHK
News Centre
CUHK develops world’s first antibiofilm liquid-bodied robot for precise eradication of implant-related biofilm infections
An international research team led by The Chinese University of Hong Kong (CUHK) has achieved a breakthrough in the field of medical microrobot. Led by Professor Zhang Li from the Department of Mechanical and Automation Engineering in CUHK’s Faculty of Engineering, the team – in partnership with Nanyang Technological University (Singapore) and the Max Planck Institute for Intelligent Systems (Germany) – has developed the world’s first antibiofilm liquid-bodied magnetic-controlled robot, introduces new features including, possessing unique viscoelastic properties that allow the robot to adapt to diverse operational environments, and along with a triple synergistic antibiofilm mechanism, paving the way for innovative solutions to combat biofilm infections. The findings have been published in the renowned international research journal Science Advances.
The challenge of biofilm infections
The World Health Organization (WHO) declared antimicrobial resistance (AMR) as one of the top ten global public health threats facing humanity in 2019, causing nearly 5 million deaths per year globally. AMR is not only related to the emergence of resistant bacterial strains but also significantly due to the formation of biofilm barriers, where bacteria adhere to surfaces and secrete substances. Medical implants inside the human body that lack of immune protection are highly susceptible to biofilm infections. Traditional antibiotic therapy struggles to penetrate biofilm barriers, while surgical removal of infected implants carries risks of secondary trauma.
The team previously developed magnetic microrobots to combat biofilm infections on implants. However, research revealed that while magnetically controlled hydrogel robots could navigate simple tubular structures, they struggled to adapt to complex surfaces such as medical stents and meshes, leaving residual biofilm. In light of these challenges, the team developed the world’s first antibiofilm magnetic-controlled liquid-bodied robot.
Two new features aiding in combating biofilm infections
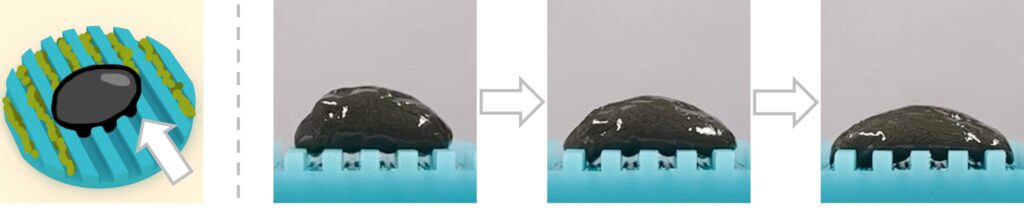
The newly developed liquid-bodied robot uses a dynamic cross-linked magnetic hydrogel with unique viscoelasticity that aid in eliminating biofilms within the human body. Professor Zhang explained: “By precisely modulating external magnetic fields, the robot can switch between viscoelastic behavioural modes. In elastic mode, it rotates, rolls and overcomes obstacles within the body. In liquid mode, it deforms into a fluidly robot to infiltrate crevices and eradicate any biofilm within them.”
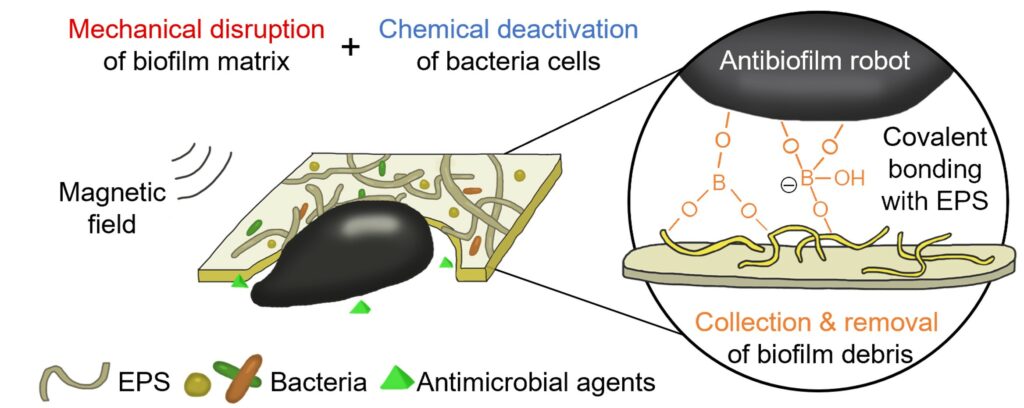
The robot also features a triple synergistic antibiofilm mechanism, including physical biofilm disruption, chemical bacteria deactivation and biofilm debris removal. First, magnetic forces transmitted through the robot’s motion mechanically disrupt biofilm structures and weaken their protective effects; then, the antimicrobial agents released by the robot target planktonic bacteria cells; and finally, the robot forms bonds with biofilm fragments, which prevents infections from recurring.
Achieved 87% effectiveness in tests aiming for future clinical applications
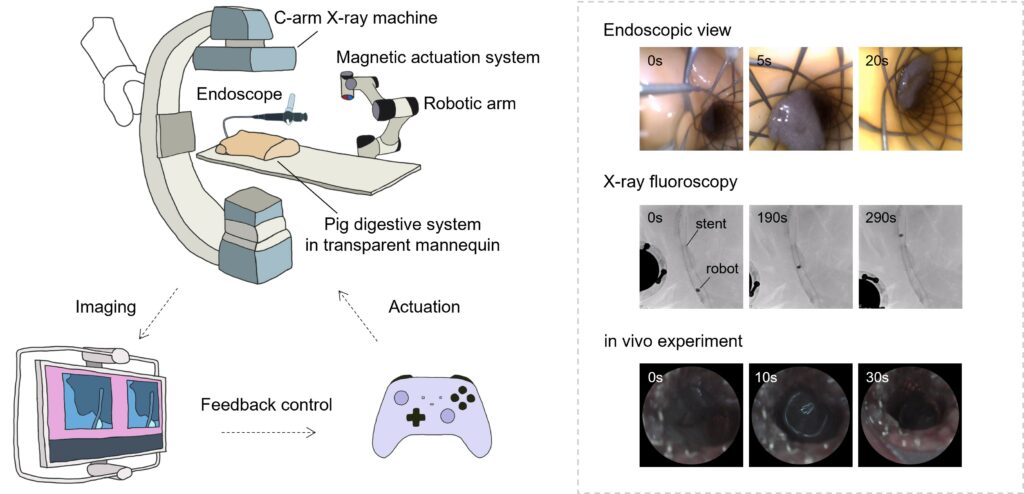
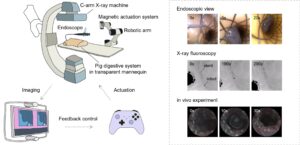
The liquid-bodied robot performed exceptionally in tests on infected medical implants. Biofilm on a 3D-structured hernia mesh was reduced by 84% after treatment, while 87% of bacteria on a metal biliary stent were killed. Professor Zhang added: “Our team pioneered dual-modality navigation using endoscopy and X-ray imaging, enabling precise control of the robot through metal stents in pig bile ducts. In a mouse model with infected stents, complete weight recovery was observed within 12 days, with a 40% reduction in inflammation indicators compared to the control group.”
“Traditional miniature robots often compromise between accessibility and driving force. This technology achieves both,” said Professor Zhang. The team is collaborating with Nanyang Technological University’s Lee Kong Chian School of Medicine to develop upgraded antibiofilm robots, with plans to advance to large animal trials and prepare for human clinical studies.
Professor Joseph Sung from Lee Kong Chian School of Medicine, a co-author of the study, commented: “Biliary biofilm infections have long been a focus of my research. When solidified biofilm completely blocks a patient’s bile duct, conventional therapies often fail. This liquid robot offers a novel solution. We aim to integrate next-generation antimicrobial agents and validate its efficacy in clinical settings.”
For the full research, please visit: https://www.science.org/doi/10.1126/sciadv.adt8213

Professor Zhang Li from CUHK’s Department of Mechanical and Automation Engineering led a collaborative research team that has successfully developed the world’s first antibiofilm liquid-bodied magnetic-controlled liquid-bodied anti-biofilm robot, paving the way for innovative solutions to combat biofilm infections.

The antibiofilm robot can transform into liquid mode, penetrating deep into crevices within a patient’s body to remove biofilms.