CUHK
News Centre
CUHK Breakthrough in Discovering the Mechanism of Plant COPII Vesicle Formation to Mitigate Abiotic Stresses
Providing New Insights into Developing Stress-resistant Crops
Plants have developed various counter mechanisms to subdue the stress caused by extreme environments such as drought and heat. For instance, some plants will undergo defoliation or close their stomata to reduce water loss and increase the chance of survival. Details of how these mechanisms were activated in the endomembrane system have still not been clear.
Recently, the Centre for Organelle Biogenesis and Function, School of Life Sciences at The Chinese University of Hong Kong (CUHK) has made a major breakthrough in identifying and functionally characterising a new population of giant COPII vesicles in response to hormone regulation and abiotic (drought) stresses in plants, with potential impact on the development of drought-tolerant crops and therefore future environmental sustainability. Results have been published in the prestigious scientific journal Nature Plants.
The endoplasmic reticulum (ER) in the endomembrane system of eukaryotic cells is the major protein synthesis factory for membrane and secreted proteins. The COPII coated vesicles are subsequently responsible for mediating protein transport from the ER to the Golgi apparatus. Precise selection of cargoes by vesicles determines the homeostasis of the cell.
Professor JIANG Liwen, Choh-Ming Li Professor of Life Sciences and Director of the Centre for Organelle Biogenesis and Function, highlighted the importance of the research project, “Climate change poses a considerable threat to global food security. Understanding the mechanisms of protein trafficking as well as organelles biogenesis and function in plants could bring us insights on boosting drought tolerance and the production yield of crops by means of bioengineering. This project focuses on the mechanism of COPII vesicles production by hormonal regulation under drought stresses and its related protein reactions.”
The research team has combined biochemical, cellular, molecular and proteomic approaches with cutting-edge electron microscopy techniques and experience gained over the past two decades to reconstitute COPII vesicles using plant-derived materials in vitro for the first time. They have identified giant COPII vesicles, twice the size of a normal COPII vesicle, induced by abscisic acid (ABA) treatment. These giant vesicles package a large number of stress-relevant membrane proteins to their destination, to trigger the mechanism and relieve the plants from stresses.
They have also revealed that the induction of giant COPII vesicles is regulated by a protein AtSar1a. In a drought stress experimental environment, the AtSar1a-lacked mutant plants, compared with their wild species, had their stomata opened wider and eventually suffered from severer water loss. Based on these new findings, the research team proposed a novel molecular mechanism underlying plant response to abiotic stresses and provided a new insight for the future development of drought-tolerant plants/crops in plant biotechnology, with potential impact on environmental sustainability.
Professor JIANG said, “We started this project almost 10 years ago in collaboration with Prof. Randy SCHEKMAN, 2013 Nobel Laureate in Physiology or Medicine, where we learnt the techniques of in vitro reconstitution. It has been very challenging because of the multiple advanced skills developed and used in studying COPII vesicles in plants for the first time. This study not only discovered a novel molecular mechanism underlying plants response to abiotic stresses, but also provided new insights for future development of drought-tolerant plants/crops in plant biotechnology, marking a new milestone in our team’s research achievement under the Areas of Excellence Scheme.”
The co-first authors of this research paper were Dr. LI Baiying and Dr. ZENG Yonglun, post-doctoral researchers of the Centre for Organelle Biogenesis and Function, CUHK. Professor JIANG and Professor GUO Yusong from the Hong Kong University of Science and Technology are the corresponding authors. The full journal paper can be read at:
https://www.nature.com/articles/s41477-021-00997-9
About the CUHK RGC-AoE Centre for Organelle Biogenesis and Function
Since joining CUHK in 2000, Professor JIANG Liwen and his team have carried out researches in plant cell biology with a number of novel findings in protein trafficking, organelle biogenesis and function, including identification of new regulators, organelle, transport vesicles and transport pathways in plants that are internationally recognised. In addition, with the support of the Research Grants Committee and CUHK, the Centre for Organelle Biogenesis and Function has established several state-of-art shared platforms for promoting collaborative research and research excellence at CUHK and in Hong Kong, including the first 3D TEM system, the first Cryo-EM/ET system with Volta phase plate (VPP) and more recently, the first Cryo-FIB and Cryo-CLEM systems. They offer the best possible means to analyse 3D morphological structures of membranous organelles and macromolecular assemblies in their native state.

A research team led by Professor JIANG Liwen, CUHK School of Life Sciences, has shed new light on the plant giant COPII vesicle formation upon stress regulation.
(From left) Professor JIANG Liwen, Professor YAO Zhong-ping, Professor GUO Yusong, Dr. LI Baiying, Miss. Zhang Wenxin, Dr. ZENG Yonglun, Dr. CAO Wenhan.
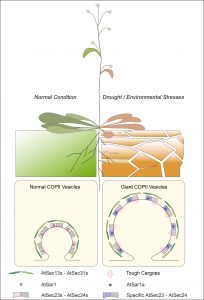
The research team conducts experiments on Arabidopsis to compare the change of COPII vesicle formation under normal condition and drought / environmental stresses. It is found that giant COPII vesicle formation is mediated by AtSar1a under stress conditions for plants to accommodate to the increased demand of large transporters or ion channels trafficking in responses to the environmental stresses.
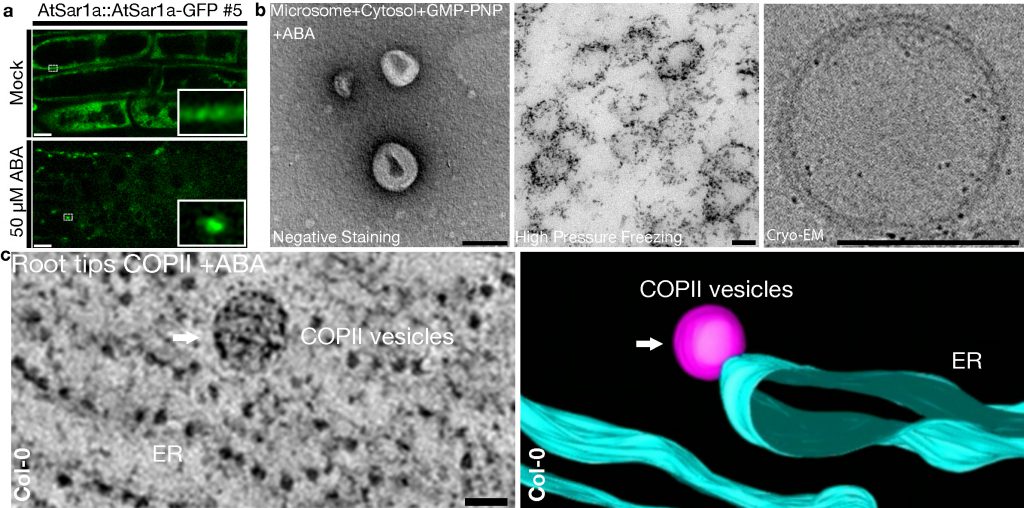
a. Plant Sar1 homolog AtSar1a is regulated by abscisic acid (ABA) to form large puncta in vivo.
b. In vitro formed COPII vesicles are observed by transmission electron microscopy (TEM) or cryogenic EM.
c. 3D-electron tomography (ET) revealed the in vivo ABA-induced ArSar1a-dependent giant COPII vesicles.