CUHK
News Centre
CU Medicine establishes Asia’s first bladder cancer organoid biobank
Pioneering a new era in precision medicine with a “test-before-treatment” approach
The Chinese University of Hong Kong’s (CUHK) Faculty of Medicine (CU Medicine) has successfully created the first Asian population-based bladder cancer patient-derived organoid (hereafter ‘‘organoid’’) biobank, which not only offers new hope for precision treatment to bladder cancer patients in Hong Kong but also marks a significant milestone in oncology research in Asia. Details of this groundbreaking achievement have been published in the internationally renowned journal Advanced Science.
Organoids overcome major challenges in treating bladder cancer
Bladder cancer is a major urological disease and among the top 10 most common cancers in the world. It is mainly divided into non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC), which have different molecular features. The disease is prone to metastasis and recurrence, posing challenges to clinical treatment. A previous study by CUHK research indicates that the average one-year recurrence rate of bladder cancer is about 35%, while the five-year recurrence rate exceeds 50%, accompanied by a high mortality rate.
Surgery and chemotherapy are first-line treatments, but surgery can easily cause various complications; while chemotherapy, the first-line treatment at advanced stages, may induce drug resistance. Region, race and genetic mutations can bring about different treatment responses, affecting patients’ recovery and survival rates.
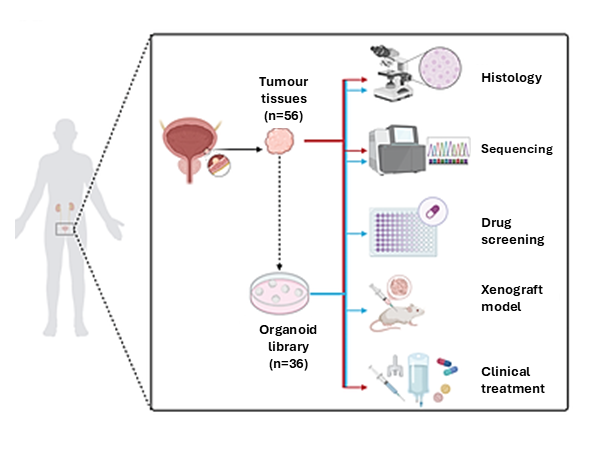
The study used tumour samples from 56 bladder cancer patients within Hong Kong’s public hospital system to establish 36 organoid models, 80% of them representing NMIBC, closely mirroring local disease characteristics. Organoids are three-dimensional cell structures cultivated in vitro using stem cells from an organ, and they function as in vitro miniature organ models that simulate partial structural composition and functions, enabling doctors to unravel disease mechanisms, study various genetic mutations and progression within tumors, and conduct drug screening. The technology opens up new horizons for bladder cancer treatments, as well as subsequent research and clinical trials, both locally and across Asia:
- More personalised treatment: the organoids retain the genomic and transcriptomic[1] features of patients’ tumour Through in vitro drug sensitivity tests, they facilitate more accurate prediction of individual responses to drugs used for chemotherapy and targeted therapies, which will prevent side effects and ineffective treatments, and ease the burden on patients.
- Faster local clinical trials: in strict adherence to Hong Kong’s public hospital protocols, this research pioneered a standardised workflow from sample collection through organoid cultivation to drug tests, laying the foundation for future organoid-guided clinical trials. This approach is expected to significantly shorten R&D cycles and reduce treatment costs.
- Cross-disease research: the research offers invaluable insights into the establishment of organoid biobanks targeting other diseases. This helps to develop Hong Kong into a regional research hub for precision medicine.
The first author of the research Dr Zhao Hong-da, a postdoctoral fellow from the Division of Urology in the Department of Surgery at CU Medicine, said: “Asian and Western populations vary significantly in genetic mutation profiles, treatment responses and survival rates. The Asian-based biobank, which is validated by xenograft animal studies and patient clinical treatment outcomes, helps local doctors provide more precise prognoses and treatments. Bespoke treatments increase the success rate of clinical trials and benefit more patients in Hong Kong and other regions in Asia.”
A “test-before-treatment” approach to drug resistance advances precision medicine development
The biobank revolutionises the drug treatment of bladder cancer. Through drug screening tests via multiple conventional chemotherapeutic and targeted therapy drugs, the organoid technology is capable of testing tumour-drug interactions in vitro rapidly. The duration of drug sensitivity tests can be shortened to one week and drug testing on humans is no longer required, significantly improving the safety and effectiveness of treatments and providing doctors with more systematic decision-making guidance. A “test-before-treatment” strategy like this is faster than traditional trial-and-error approaches and widens the application from laboratory research to local clinical application.
By preserving Hong Kong’s unique genetic profiles, the local biobank also ensures that research outcomes align well with local needs, integrating Hong Kong-based data with Hong Kong-based treatments in a one-stop model, and laying a scientific foundation for precision medicine.
Accelerating clinical translation
Organoid technology bridges fundamental research and clinical applications, deepening insights into tumour microenvironments and the potential mechanisms of drug resistance. The achievement opens new avenues for new drug research and development, as well as precision medicine targeting the microenvironments of bladder tumours.
Dr Jeremy Teoh Yuen-chun, the corresponding author and an Associate Professor from the Division of Urology in the Department of Surgery at CU Medicine, said: “This biobank offers great promise for the advancement of innovative therapies for bladder cancer, while offering valuable insights and a reference point for the treatment of other cancers. Quicker access to a personalised treatment blueprint will be possible through minimally invasive biopsies, enabling truly personalised precision medicine under a one-patient-one-strategy regime.”
[1] Transcription refers to the process by which genetic information in DNA is transcribed into RNA. The transcriptome resembles a cell’s activity list, recording which genes whose expressions are turned on by hormones or by growth factors and transcribed.
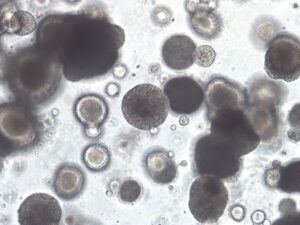
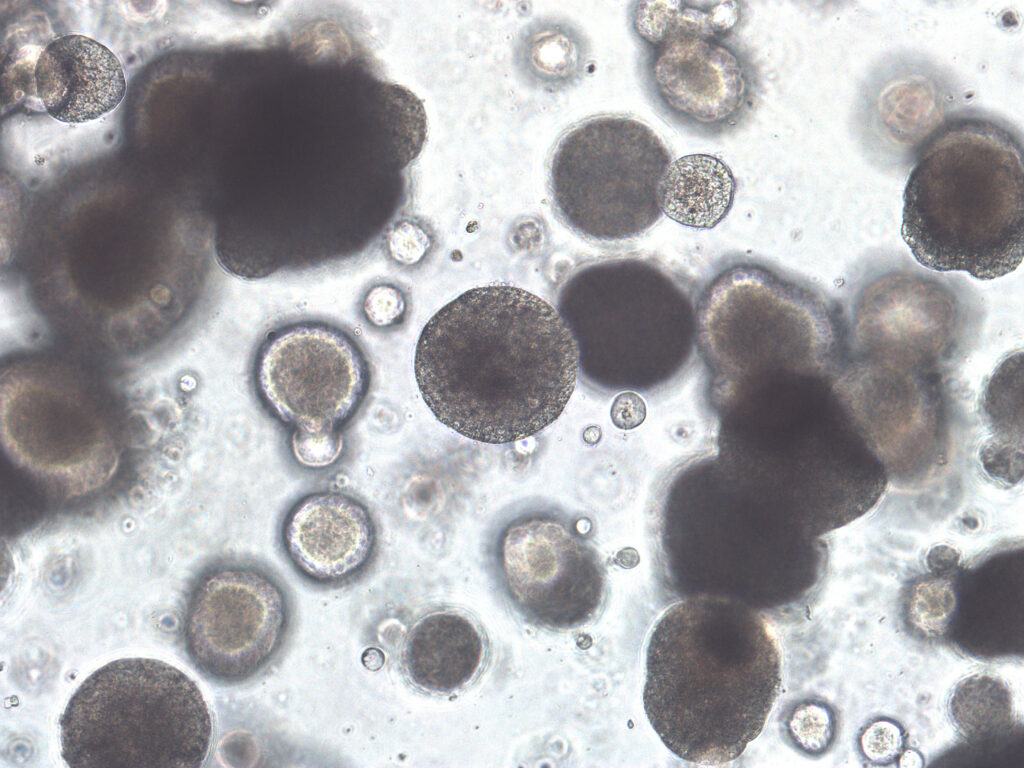
CU Medicine has established Asia’s first Asian population-based bladder cancer patient-derived organoid biobank which consists of 36 organoid models developed by utilising tumour samples from 56 bladder cancer patients within Hong Kong’s public hospital system. As three-dimensional cell structures cultivated in vitro, organoids can simulate partial structural composition and functions, enabling doctors to unravel disease mechanisms, study various genetic mutations and progression within tumours, and conduct drug screening.
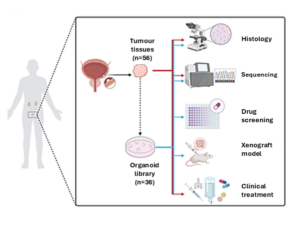
The organoid biobank supports the ‘‘test-before-treatment’’ approach by shortening the duration of drug sensitivity tests while saving the need of drug testing on humans, significantly enhancing the safety and effectiveness of treatments and providing doctors with more systematic guidance for decision-making during clinical treatment.

Featured are research team members: (from left) Dr Wu Ding-lan, Assistant Professor, Professor Ng Chi-fai, Tzu Leung Ho Professor of Urology, Dr Zhao Hong-da, the first author of the research and the postdoctoral fellow and Dr Jeremy Teoh Yuen-chun, the corresponding author and Associate Professor. All of them are from the Division of Urology, Department of Surgery at CU Medicine.