CUHK
News Centre
CU Medicine establishes an internationally accredited biobank: A prerequisite for Hong Kong to be the hub for new drug development in the Greater Bay Area
The Chinese University of Hong Kong (CUHK)’s Faculty of Medicine (CU Medicine) has successfully established an internationally accredited biobank. CU-Med Biobank will be a driving force for the development of biomedicine in the Guangdong-Hong Kong-Macao Greater Bay Area, and lead Hong Kong to become an important biomedical research hub in the region.
The biobank was built at the Prince of Wales Hospital in 2019. After years of dedicated effort, CU Medicine is honoured to receive international accreditation [ISO 20387:2018(en)] from A2LA, which is an endorsement of its high standards. Together with one in South Korea, it is one of only two biobanks that fulfil the required international standards in the Asia-Pacific region. The accreditation proves that Hong Kong is qualified to become a new drug research and development centre in the Greater Bay Area.
Possession of an internationally accredited biobank can accelerate research and development of new drugs
To develop new drugs or pioneer new diagnostic technologies requires both software and hardware, and one prerequisite is possessing of a biobank. A biobank is an essential facility for the systematic storage of clinical data and bio-specimens. It is not just a repository for sample storage, but also includes a set of strict management systems for handling diverse biological samples and precise multi-omics analysis.
Professor Francis Chan, Dean of Medicine and Head of the Steering Committee of CU-Med Biobank at CU Medicine, said, “The standard of biobanking determines not only the quality of academic research but also the potential capability for new drug development. The threshold for becoming an internationally accredited biobank is very high; our achievement can help attract collaborations from top global scientific research teams and multinational pharmaceutical companies. This international accreditation will provide CU Medicine with valuable credibility that will allow us to work with the Hospital Authority and transform Hong Kong into the hub for research and new drug development in the Guangdong-Hong Kong-Macao Greater Bay Area.”
Professor Ronald Ma, Co-Head of the Executive Committee of CU-Med Biobank, and Professor at the Department of Medicine and Therapeutics at CU Medicine, said, “Genomic research has rapidly developed over the past two decades due to the emerging trend of precision medicine and personalised medicine. A biobank is therefore considered a precious asset for humans because it not only stores a vast amount of genetic data, but also biospecimens with the potential to generate a variety of other biomarker information, and therefore great potential to advance medical research.”
The international accreditation process is rigorous which took nearly six years to attain from preparation
CU Medicine has aimed to achieve international standards since the initial preparation stage for establishing the biobank. After five years of system establishment, operation, and preparation, plus a review process lasting over four months, the team was officially awarded the ISO 20387:2018(en) accreditation this year.
Dr Terry Or, Scientific Officer of CU-Med Biobank, explained, “The CU-Med Biobank has strictly adhered to regulations in four major areas: sample acquisition, processing, storage, and distribution, and from there we attained the international accreditation. This qualification will help promote large-scale international research cooperation, attract local, mainland and overseas scientific research teams and pharmaceutical research institutions to collaborate, and thus enhance the efficiency and level of biomedical research.”
Potentials of CU-Med Biobank seen in breast cancer, diabetes, and gut microbiome research
In the past few years, various research teams at CU Medicine have started using the service of CU-Med Biobank for large-scale genomic research, including breast cancer studies. One research team has built a Breast Cancer Biobank using the CU-Med BioBank, which will store tens of thousands of biological samples, and histology, genetic and multi-omics data.
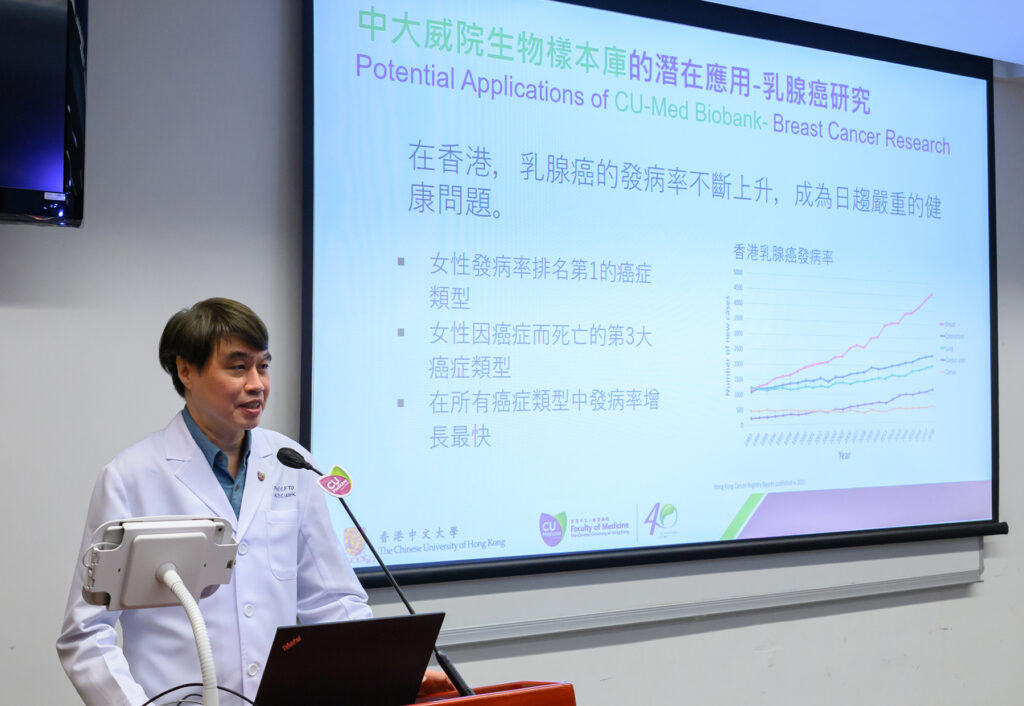
Professor To Ka-fai, Co-Head of the Executive Committee of CU-Med Biobank, and Chairman of the Department of Anatomical and Cellular Pathology at CU Medicine said, “Breast cancer ranks first among all cancer types affecting women in Hong Kong. The establishment of a Breast Cancer Biobank can facilitate large-scale multi-omics analysis, comprehensive research on the long-term therapeutic effects of breast cancer treatment, and screening of effective prognostic markers and predictive markers. The abundant sample resources can also aid drug development.”
The CU Medicine diabetes team, which has extensive experience in genomic research, has successfully identified multiple genetic markers and other biological markers related to diabetes and its complications over the past decade by establishing the Hong Kong Diabetes Biobank. The findings will help identify patients at risk of developing diabetes, as well as those with diabetes who are at high risk of kidney disease or cardiovascular disease.
The Microbiota I-Centre of CU Medicine has established ’the world’s largest 100k mother-infant cohort since 2019, which positions Hong Kong as a leading hub in the new microbiota industry in the Asia-Pacific region.

CU Medicine has successfully established an internationally accredited biobank CU-Med Biobank. Together with one in South Korea, it is one of only two biobanks that fulfil the required international standards in the Asia-Pacific region under the Asia Pacific Accreditation Cooperation (APAC). The biobank is a driving force for the development of biomedicine in the Guangdong-Hong Kong-Macao Greater Bay Area, and lead Hong Kong to become an important biomedical research hub in the region.
The photo shows members of the CU-Med Biobank, including (from the 3rd from left) Professor To Ka-fai and Professor Ronald Ma, Co-Heads of the Executive Committee of CU-Med Biobank; Professor Francis Chan, Dean of Medicine and Head of the Steering Committee of CU-Med Biobank; and Dr. Terry Or, Scientific Officer of CU-Med Biobank.

Professor Francis Chan says CU Medicine will work with the Hospital Authority to transform Hong Kong into the hub for research and new drug development in the Guangdong-Hong Kong-Macao Greater Bay Area.

Professor Ronald Ma explains that biobank not only stores a vast amount of genetic data, but also biospecimens with the potential to generate a variety of other biomarker information, and therefore great potential to advance genomic research.
Professor To Ka-fai says research team has built a Breast Cancer Biobank using the CU-Med BioBank, which can facilitate large-scale multi-omics analysis, comprehensive research on the long-term therapeutic effects of breast cancer treatment, and screening of effective prognostic markers and predictive markers, also aid drug development.

Dr Terry Or says CU-Med Biobank has a set of strict management systems for handling diverse biological samples and precise multi-omics analysis. The team will continuously review and upgrade its facility and workflow to optimise the operation.

The CU-Med Biobank has strictly adhered to regulations in four major areas: sample acquisition, processing, storage, and distribution.

The CU-Med Biobank has strictly adhered to regulations in four major areas: sample acquisition, processing, storage, and distribution.