CUHK
News Centre
CUHK develops novel retrievable nanorobots for targeted and enhanced thrombolysis
potentially saving stroke patients from brain damage
A cross-disciplinary research team from The Chinese University of Hong Kong (CUHK) has developed magnetic tissue plasminogen activator (tPA)-anchored nanorobots (tPA-nbots) to treat ischemic stroke. The novel technology exhibits a thrombolysis rate 5 to 20 times faster than traditional treatment and capabilities in recanalising more distal and smaller branches. It demonstrates potential to benefit patients by reducing brain damage and minimising side effects. The team also succeeded in using laser speckle contrast imaging (LSCI) guidance for real-time tracking and delivery of nanorobots and instant monitoring the bloodstream, providing a novel approach for nanorobots-based endovascular intervention therapy. Study results have been published in the international journals Science Advances and Science Robotics.
Timely and precise intervention increases the potential for stroke recovery
Stroke ranks as the second-leading global cause of death and is the most common cause of adult long-term physical or cognitive disability. tPA is a drug commonly used to dissolve occlusive clots (thrombolysis), restoring blood flow in patients with acute ischemic stroke. However, the unfocused diffusion of high-dose tPA over the whole body undermines the effectiveness of clot lysis and poses a risk of systemic and brain hemorrhage during treatment.
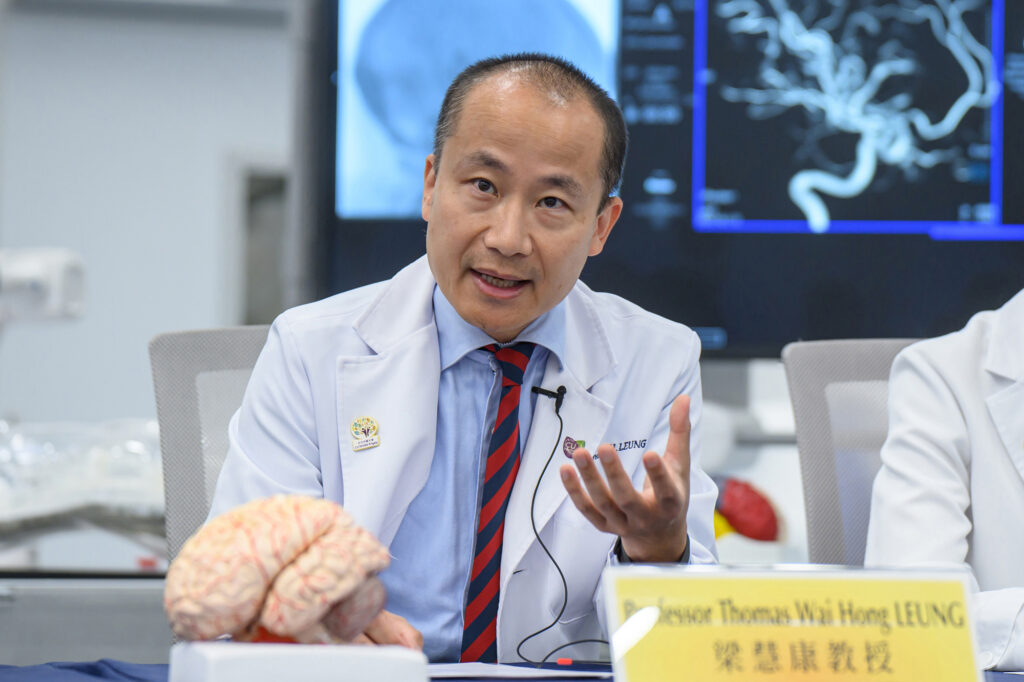
Professor Thomas Leung Wai-hong, Head of the Division of Neurology and Lee Quo Wei Professor of Neurology in CU Medicine’s Department of Medicine and Therapeutics, emphasised the importance of prompt and timely re-opening of occluded blood vessels, resuming blood perfusion to the ischemic brain. He stated, “Without treatment, brain cells die at an alarming rate of 1.9 million every minute from stroke onset. While we can now access the main stem of the brain arteries and remove the occlusive clots through catheters, the technological bottleneck is recanalising more distal and smaller branches that are equally vital in preserving brain function. One key challenge is how we can accomplish this goal in a safe and effective manner.”
Fast recanalisation by retrievable tPA-nbots for enhanced thrombolysis
To address these challenges, a research team with members from the Department of Mechanical and Automation Engineering at the Faculty of Engineering, the Department of Imaging and Interventional Radiology, and the Department of Medicine and Therapeutics at CU Medicine jointly developed retrievable magnetic tPA-nbots. By deploying a tPA-nbot-loaded catheter to the thrombus site, the tPA-nbot microswarm is remotely actuated to the blood clot within vessels of submillimetre size, initiating the tPA-mediated thrombolysis process. After the blood clot is lysed, the tPA-nbots will be guided back for retrieval, ensuring biomedical safety.
Professor Zhang Li from the Department of Mechanical and Automation Engineering explained, “The size of tPA-nbots (~300 nm) allows them to be navigated to the thrombus site within the narrow distal blood vessels. The enhanced, localised delivery can prevent high-dose tPA from being circulated in the body, reducing the possibility of systemic and brain hemorrhage. Even with the reduced dosage, the tPA-nbots with both mechanical and chemical etching exhibit a thrombolysis rate 5 to 20 times faster than traditional treatment and save abundant recanalisation time, potentially saving patients from heavy brain damage.”
Professor Simon Yu Chun-ho, Emeritus Professor in the Department of Imaging and Interventional Radiology at CU Medicine, explained, “This novel treatment system can potentially deploy nanorobots to the exact location of the thrombus in peripheral and small arteries, which are hardly accessible with catheters alone. Although many technical issues remain to be solved before this technology can be applied in clinical practice, I believe we have taken an important step in the right direction.”
Tracking and navigation of microswarm under LSCI to ensure delivery efficiency and biomedical safety
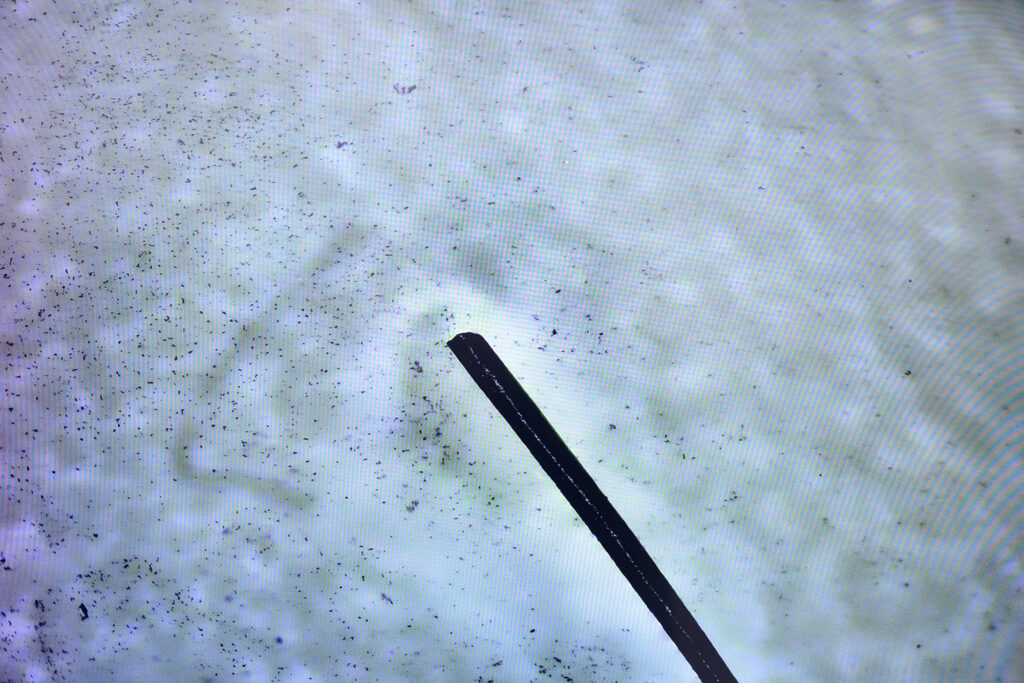
To overcome the limitations of current imaging methods in imaging size and spatial-temporal resolution, the team has proposed a strategy using LSCI to enable in vivo real-time tracking and navigation of nanorobots in the endovascular system. LSCI can monitor the changes in the bloodstream within the area of interest and assess reperfusion status after an ischemic stroke. It ensures delivery efficiency and biomedical safety in complex vascular environments, allowing monitoring and analysis of the thrombolysis process, including changes to the state of the blood clot.
Dr Bonaventure Ip Yiu-ming, Assistant Professor in the Department of Medicine and Therapeutics at CU Medicine, commented, “Real-time monitoring of the behaviour of nanorobots under physiological conditions is a crucial step to prove their safety and effective delivery. By employing LSCI, we were able to observe and track the movement of nanorobots in both stagnant and flowing blood environments in vessel models, placenta and small animals. This visualisation marks a critical milestone in refining the dose of nanorobots and the distance, strength and angulation of the magnetic field applied. It is essential to carefully optimise these parameters before implementing the technology in humans.”
Professor Tony Chan Kai-fung, Research Assistant Professor of the Chow Yuk Ho Technology Centre for Innovative Medicine, added, “The proposed tPA-nbots with high spatial precision provide a promising robotic tool to enhance thrombolysis efficiency and safety, while side effects and treatment time are greatly reduced. It shows the great potential for clinical translation.”
The original studies can be accessed here:
Science Advances: https://doi.org/10.1126/sciadv.adk8970
Science Robotics: https://doi.org/10.1126/scirobotics.adh1978

CUHK has developed magnetic tissue plasminogen activator (tPA)-anchored nanorobots (tPA-nbots) to treat ischemic stroke. It demonstrates potential to benefit patients by reducing brain damage and minimising side effects. The team also succeeded in using laser speckle contrast imaging (LSCI) guidance for real-time tracking and delivery of nanorobots and instant monitoring the bloodstream, providing a novel approach for nanorobots-based endovascular intervention therapy.
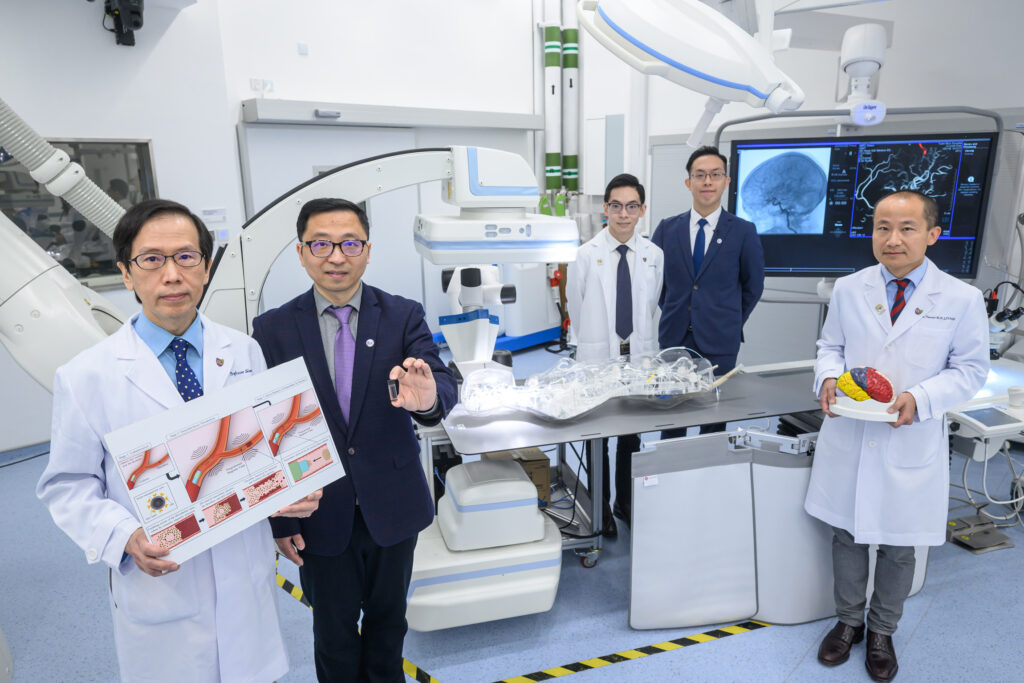
(From left) Professor Simon Yu Chun-ho, Emeritus Professor in the Department of Imaging and Interventional Radiology at CU Medicine; Professor Zhang Li, Professor in the Department of Mechanical and Automation Engineering, CUHK; Dr Bonaventure Ip Yiu-ming, Assistant Professor in the Department of Medicine and Therapeutics at CU Medicine; Professor Tony Chan Kai-fung, Research Assistant Professor of the Chow Yuk Ho Technology Centre for Innovative Medicine; and Professor Thomas Leung Wai-hong, Head of the Division of Neurology and Lee Quo Wei Professor of Neurology in CU Medicine’s Department of Medicine and Therapeutics
Professor Thomas Leung emphasises the importance of prompt and timely re-opening of occluded blood vessels, resuming blood perfusion to the ischemic brain after stroke. While it can now access the main stem of the brain arteries and remove the occlusive clots through catheters, the technological bottleneck is recanalising more distal and smaller branches that are equally vital in preserving brain function. One key challenge is how to accomplish this goal in a safe and effective manner.

Professor Zhang Li explains that the tPA-nbots can be navigated to the thrombus site within the narrow distal blood vessels. The enhanced, localised delivery can prevent high-dose tPA from being circulated in the body, reducing the possibility of systemic and brain hemorrhage, and potentially saving patients from heavy brain damage.
Professor Simon Yu explains that the novel treatment system can potentially deploy nanorobots to the exact location of the thrombus in peripheral and small arteries, which are hardly accessible with catheters alone.

Dr Bonaventure Ip says the research team was able to observe and track the movement of nanorobots in both stagnant and flowing blood environments by employing LSCI. The visualisation marks a critical milestone in refining the dose of nanorobots and the distance, strength and angulation of the magnetic field applied.