CUHK
News Centre
CUHK physics team discovers a new surprising form of bacterial teamwork that builds ‘bacterial canals’ to transport materials over long distances
A study led by a research team from the Department of Physics at The Chinese University of Hong Kong (CUHK) has discovered a surprising form of bacterial teamwork: the bacteria can spontaneously build channel networks for effective delivery of materials over long distances, like human-made canals, and have therefore been named “bacterial canals”. This discovery challenges scientists’ belief that long-range material transport in the microbial world was inefficient. The canals also help bacteria to eradicate competitors. The discovery suggests a novel principle to engineer synthetic microbial communities in biomedicine and the environment, and furthers understanding of the pathogenic mechanism of bacteria. The findings have recently been published in the scientific journal eLife.
Discovering the formation of bacterial canals in a common bacterium
Long-range material transport is essential to maintaining the physiological functions of multicellular organisms such as animals and plants. By contrast, material transport in microbial world is carried out by molecular diffusion, and is often short-range, with low efficiency over longer ranges. Professor Wu Yilin and his PhD students and first authors of the paper, Li Ye and Liu Shiqi at CUHK’s Department of Physics discovered that bacterial colonies can actively regulate themselves for effective material transport over long distances, like animals.
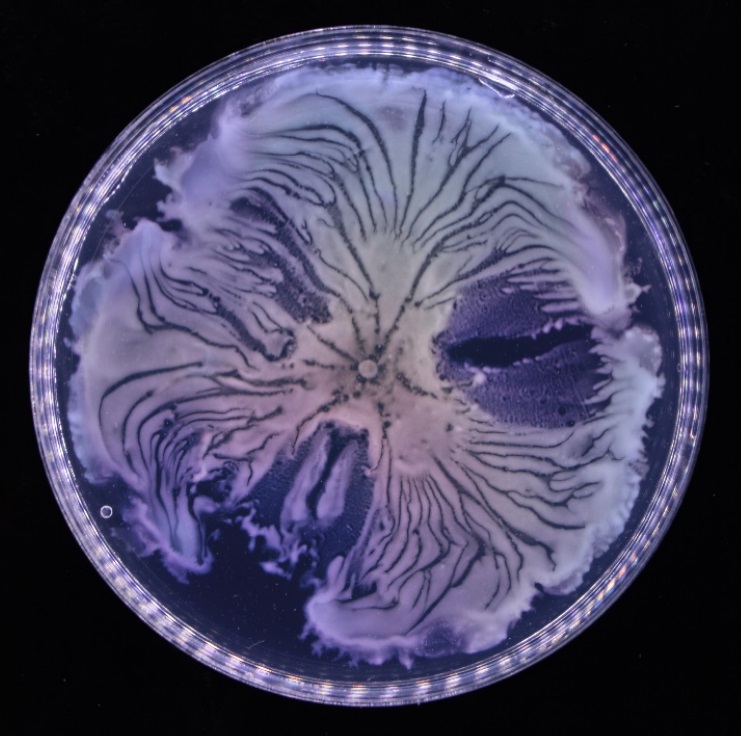
The team discovered this novel form of bacterial teamwork in colonies of Pseudomonas aeruginosa, a bacterium often found in the natural environment and wound infections. They found that a number of open fluid channels, with a width of tens to hundreds of microns, spontaneously develop in a bacterial colony. The fluid channels support the transport of cells and outer membrane with a peak speed of 450 microns per second, more than 10 times the typical swimming speed of bacteria. These channels look like human-made canals for cargo transport, and the team calls them “bacterial canals”.
By using multiscale microscopy, particle tracking and physical modelling, the team has elucidated the mechanism of bacterial canal formation. They found that the development of bacterial canals is essentially driven by interfacial mechanics and the instability of complex fluids. Bacterial cells can actively produce “surfactants”, molecules that lower the surface tension of liquids, like those commonly found in cleaning detergents. While the colony becomes a complex fluid consisting of cells and surfactants, the surfactants diffuse away from the colony, leading to a lower surface tension inside the colony than outside. The surface tension difference pulls the colony outwards. During this pulling process, a number of banded flow regions are formed, which become bacterial canals when stable. The team also developed a mathematical model that incorporates the interfacial mechanics, material transport and cell-cell communication to understand the spatial and temporal changes of material transport in bacterial canals.
Helping to eradicate superbugs and advancing the development of synthetic biology
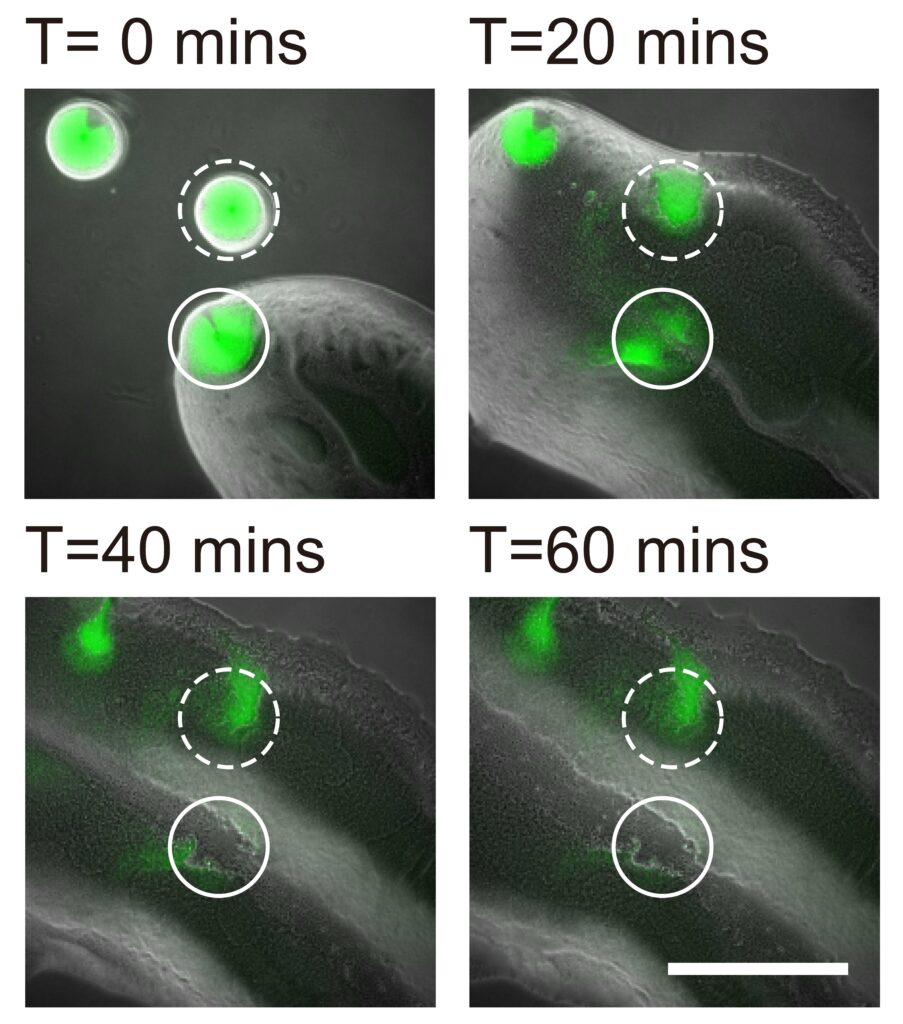
In collaboration with Professor Yang Liang from the School of Medicine at the Southern University of Science and Technology in Shenzhen, the team also demonstrated that the bacterial canals help to eradicate competitors of Pseudomonas aeruginosa, such as Staphylococcus aureus, a human pathogen that often causes skin and soft tissue infections, and is known for its ability to develop tolerance to multiple antibiotic drugs, with some of its members known as “superbugs”. The study provides a better understanding of the competition or coexistence between pathogenic bacteria, as well as bacterial dispersal during pathogenesis.
Professor Wu remarked, “The studies deepen the understanding of living matter physics, and demonstrate that interfacial fluid mechanics can be exploited by primitive lifeforms to drive large-scale material transport. It provides a new strategy to design self-organised functions in synthetic microbial communities, advancing the development of bioengineering, biomaterials and synthetic biology.”
The CUHK research team was supported by the Hong Kong Research Grants Council, the National Ministry of Science and Technology, and the CUHK Research Committee.
The full text of the paper can be found at: https://doi.org/10.7554/eLife.79780

Self-organised bacterial canals developed in a colony of the bacterium Pseudomonas aeruginosa. The canals are shown as darker lines or stripes in the colony, where fluid flows move from centre to edge at a speed of hundreds of microns per second.
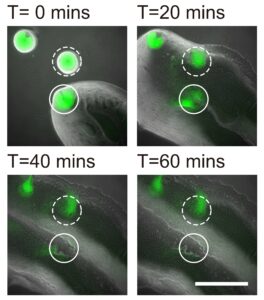
The Staphylococcus aureus colony is eradicated by the canals of Pseudomonas aeruginosa. An S. aureus colony (green, enclosed by the solid line) that encountered a canal was eradicated within an hour, while one (enclosed by the dashed line) outside the canal but still in contact with P. aeruginosa colony retained about 50% of its biomass.